Chapter: Biochemical Pharmacology : G protein-coupled receptors
Cytotoxic catecholamine analogs
Cytotoxic catecholamine
analogs
Like DOPA and methyldopa,
6-hydroxydopa (Figure 10.20a) finds its way into catecholaminergic nerve
ter-minals; it is decarboxylated inside the cell to 6-hydroxy-dopamine. The
latter compound, however, does not act as a false transmitter. Instead, it acts
as an inhibitor of the mito-chondrial respiratory chain, apparently by binding
to com plex I (NADH dehydrogenase). This leads to degeneration of the
norepinephrine- or dopaminergic neurons. When applied to newborn rats, it
largely destroys the sympathetic nervous system (both its central and
peripheral parts), and it is being used for this purpose in experimental
research.
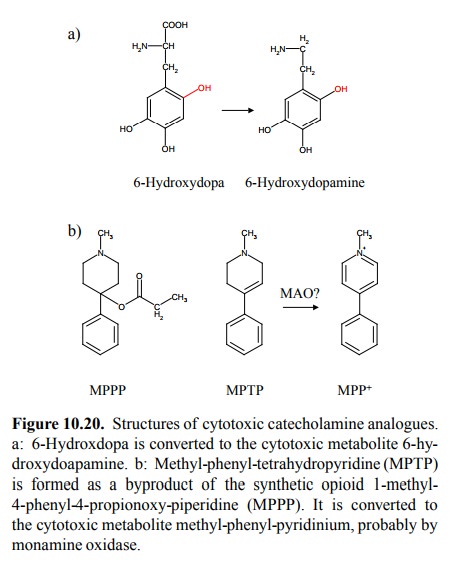
A drug
with a similar mode of action is Methyl-phenyl-tetrahydropyridine (MPTP; Figure
10.20b). This substance is is converted (apparently by monoamine oxidase, which
also oxidizes the regular catecholamine transmitters) to
1-Methyl-4-phenyl-pyridinium (MPP). MPP is again an inhibitor of mitochondrial
NADH dehydrogenase. Since MPTP enters the neurons through the dopamine reuptake
transporter, it acts selectively on dopaminergic cells and, accordingly, gives
rise to a drug-induced form of Parkin-son's disease. Of course, MPTP is not
being used in clin-ical medicine. It is formed as a by-product in the
synthe-sis of 1-methyl-4-phenyl-4-propionoxy-piperidine (MPPP, Figure 10.20b),
which in turn has a morphine-like mode of action. The poisonous action of MPTP
was discovered ac-cidentally when drug addicts suddenly developed the symp-toms
of Morbus Parkinson after partaking of a batch of MPPP from an illegally (and,
it would seem, unprofession-ally) operated laboratory that contained an
exceptionally high proportion of MPTP. MPTP has, however, subsequent-ly found application
in experimental research on the latter disease with animals.
Related Topics