Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
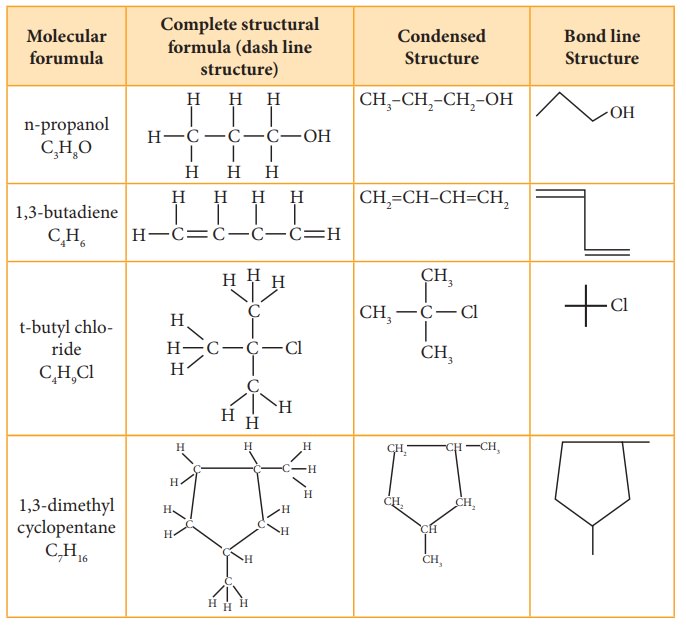
Structural representation of organic compounds
Structural
representation of organic compounds
Molecular
formula of a compound is the simplest, least informative representation,
showing the ratio of atoms present. The structure of an organic compound can be
represented using any one of the below mentioned methods.
1.
Lewis structure or dot structure,
2.
Dash structure or line bond structure,
3.
Condensed structure
4.
Bond line structure
We
know how to draw the Lewis structure for a molecule. The line bond structure is
obtained by representing the two electron covalent bond by a dash or line (-)
in a Lewis structure. A single line or dash represents single σ covalent bond,
double line represents double bond (1σ bond, 1π bond) and a triple line
represents triple bond (1σ bond, 2π bond). Lone pair of electrons on
heteroatoms may or may not be shown. This represents the complete structural
formula.![]()
![]()
This
structural formula can be further abbreviated by omitting some or all of the
dashes representing covalent bonds and by indicating the number of identical
groups attached to an atom by a subscript. The resulting expression of the
compound is called a condensed structural formula.
For
further simplification, organic chemists use another way of representing the
structures in which only lines are used. In this type of representation of
organic compounds, carbon and hydrogen atoms are not shown and the lines
representing carbon-carbon bonds are shown in a zigzag fashion. The only atoms
specifically written are oxygen, chlorine, nitrogen etc. These representations
can be easily understood by the following illustration.
Molecular models
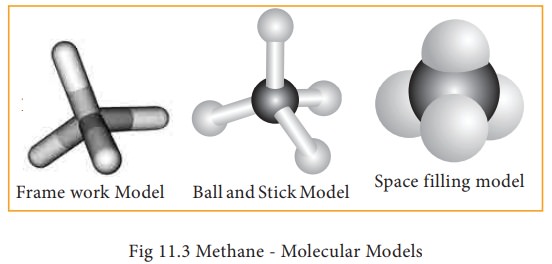
Molecular
models are physical devices that are used for a better visualisation and
perception of three dimensional shapes of organic molecules. These are made of
wood, plastic or metal and are commercially available. (i) Frame work model
(ii) Ball and stick model & (iii) space filling model. In the frame work
model only the bonds connecting the atoms themselves are shown. This model
emphasizes the pattern of bonds of a molecule while ignoring the size of the
atom. In the ball and stick model, both the atoms and the bonds are shown. Ball
represent atoms and the stick a bond. Compounds containing C=C can be best
represented by using springs in place of sticks and this model is termed as
ball and spring model. The space filling model emphasizes the relative size of
each atom based on its vander-waals radius.
Three dimensional representation of organic molecules:
The
simplest convention is solid and dashed wedge formula in which 3-D image of a
molecule can be perceived from two dimensional picture. In this representation
a tetrahedral molecule with four atoms or group a,b,c and d bonded to it can be
represented by a wedge formula as follows. A solid wedge ( ![]() ) (or a
heavy line) is used to indicate a bond projecting above the plane of the paper
and the dashed wedge (
) (or a
heavy line) is used to indicate a bond projecting above the plane of the paper
and the dashed wedge ( ) (or a dashed line) is used to depict the bond
below the plane. The bonds lying in the plane of the paper are shown by normal
lines.
) (or a dashed line) is used to depict the bond
below the plane. The bonds lying in the plane of the paper are shown by normal
lines.

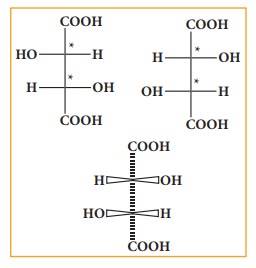
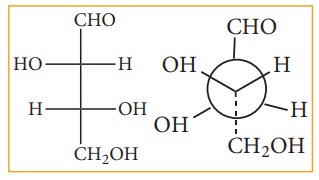
Fisher projection formula:
This
is a method of representing three dimensional structures in two dimension. In
this method, the chiral atom(s) lies in the plane of paper. The horizontal
substituents are pointing towards the observer and the vertical substituents
are away from the observer. Fisher projection formula for tartaric acid is
given below.
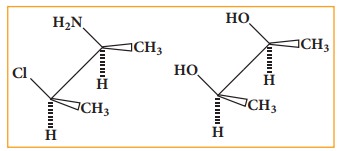
Sawhorse projection formula:
Here
the bond between two carbon atoms is drawn diagonally and slightly elongated.
The lower left hand carbon is considered lying towards the front and the upper
right hand carbon towards the back. The Fischer projection inadequately
portrays the spatial relationship between ligands attached to adjacent atoms.
The sawhorse projection attempts to clarify the relative location of the
groups.
Newman projection formula:
In
this method the molecules are viewed from the front along the carbon-carbon
bond axis. The two carbon atom forming the σ bond is represented by two
circles. One behind the other so that only the front carbon is seen. The front
carbon atom is shown by a point where as the carbon lying further from the eye
is represented by the origin of the circle. Therefore, the C-H bonds of the
front carbon are depicted from the circle while C-H bonds of the back carbon
are drawn from the circumference of the circle with an angle of 120º to each
other.
Related Topics