Equation, Procedure, Calculation, Example - Estimation of phosphorus | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Estimation of phosphorus
Estimation of
phosphorus:
Carius method:
A known mass of the organic compound (w) containing phosphorous is heated with fuming
HNO3 in a sealed tube where C is converted into CO2 and H
to H2O. phosphorous present in organic compound is oxidized to
phosphoric acid which is precipitated, as ammonium phosphomolybdate by heating
with Conc. HNO3 and then adding ammonium molybdate.
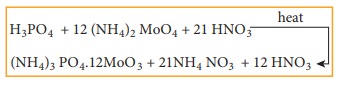
The
precipitate of ammonium phosphomolybdate thus formed is filtered washed, dried
and weighed.
In
an alternative method, the phosphoric acid is precipitated as
magnesium-ammonium phosphate by adding magnesia mixture (a mixture containing
MgCl2, NH4Cl and ammonia) This ppt is washed, dried and
ignited to get magnesium pyrophosphate which is washed, dried a weighed. The
following are the reaction that takes place.
By
knowing the mass of the organic compound and the mass of ammonium phosphomolybdate
or magnesium pyrophosphate formed, the percentage of P is calculated.
Mass
of organic compound is wg
Weight
of ammonium
phosphomolybdate = x g
Weight
of magnesium pyrophosphate = y g
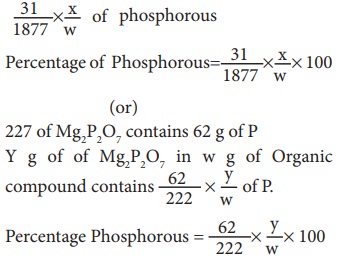
Mole
mass of (NH4)3PO4.12MoO3is =1877g
[3
x(14 + 4) + 31 +4(16)] + 12 (96+3x16)
Molar
mass of Mg3P2O7 is 222 g
(2x24)
+ (31x2) + (7x16)
1877g
of (NH4)3PO4.12MoO3contains 31g of
P
Xg
of(NH4)3PO4.12 MoO3 in w g of
organic compound contains
Example 4:
0.24 g of organic
compound containing phosphorous gave 0.66 g of Mg2P2O7
by the usual analysis. Calculate the percentage of phosphorous in the compound
Weight
of an organic compound = 0.24 g
Weight
of Mg2P2O7 = 0.66 g
222
g of Mg2P2O7 contains 62 g of P
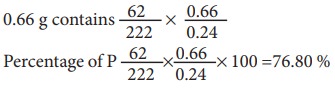
Related Topics