Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Brief questions and answers: Fundamentals of Organic Chemistry
Fundamentals of Organic Chemistry
Answer the following questions
31. Give the general characteristics of organic compounds?
●
They are convalent compounds of carbon.
●
They are insoluble in water and readily soluble in organic solvent such as
benzene, toluene, ether, chloroform etc.......
●
Many of the organic compounds are inflammable (except CCl4)
●
They possess low boiling and melting points due to their convalent nature.
●
Organic compounds are characterised by functional groups. A functional group is
an atom or a specific combination of bonded atoms that react in a
characteristic way, irrespective of the organic molecule in which it is
present.
●
They exhibit Isomerism.
●
They are characterised by homologous series.
32. Describe the classification of organic compounds based on their structure.
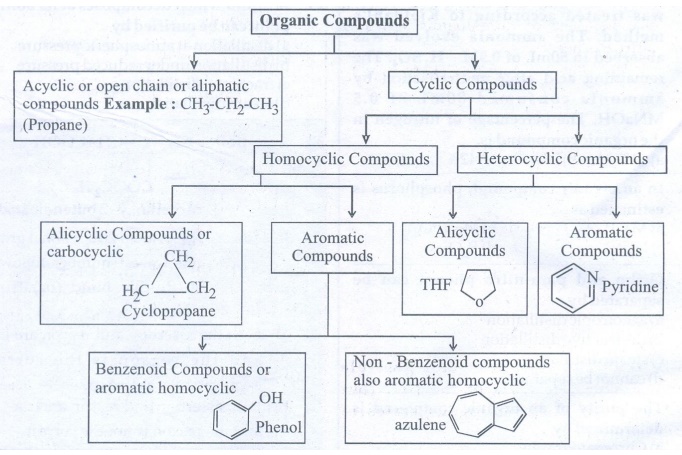
33. Write a note on homologous series.
Homologous
series : A series of organic compounds each containing a characteristic
functional group and the successive members differ from each other in moleculr
formula by a CH2 group is called homologous series. Eg Alkanes :
Methane (CH4), Ethane (C2H6), Propane (C3H8)
etc...
Alcohols
:
Methanol (CH3OH), Ethanol (C2H5OH), Propanol
(C3H7OH) etc...
●
Compounds of the homologous series are represented by a general formula Alkanes
CnH2n+ 2 Alkenes CnH2n , Alkynes CnH2n-
2 and can be prepared by general methods.
●
They show regular graduation in physical properties but have almost similar
chemical property.
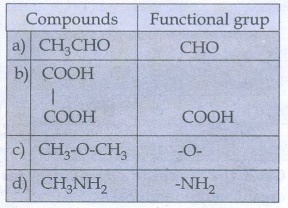
34. What is meant by a functional group? Identify the functional group in the following compounds.
(a) acetaldehyde
(b) oxalic acid
(c) di methyl ether
(d) methylamine
a)
acetaldehyde
b)
oxalic acid
c)
di methyl ether
d)
methylamine
A
functional group is an atom or a specific combination of bonded atoms that
react in a characteristic way irrespective of the organic molecule in which it
is present.
35. Give the general formula for the following classes of organic compounds
a) Aliphatic monohydric alcohol
b) Aliphatic ketones.
c) Aliphatic amines.
a)
Aliphatic monohydric alcohol: R-OH
b)
Aliphatic ketones: 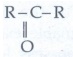
c)
Aliphatic amines: R - NH2
36. Write the molecular formula of the first six members of homologous series of nitro alkanes.
1.
CH3NO2
2.
CH3-CH2-NO2
3.
CH3 - CH2 - CH2 - NO2
4. 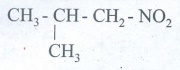
5.
CH3 - CH2 - CH2 - CH2 NO2
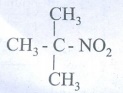
6. 
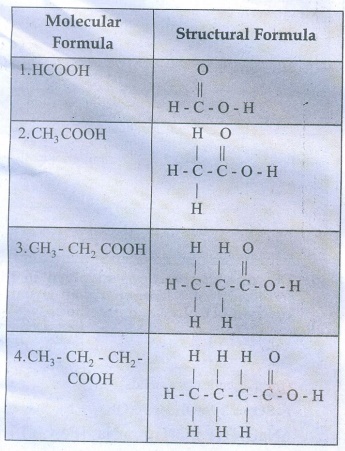
37. Write the molecular and possible structural formula of the first four members of homologous series of carboxylic acids.
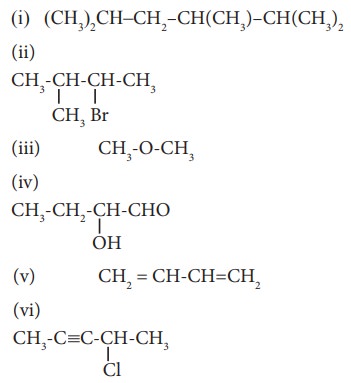
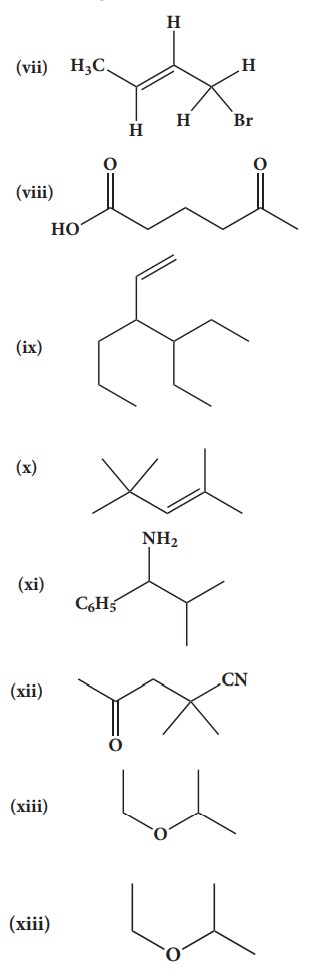
38. Give the IUPAC names of the following compounds.
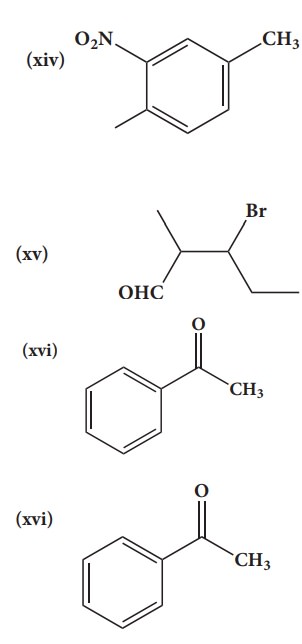
Solution:
(i) 2,3,5-trimethylhexane
(ii) 2-bromo-3-methylbutane
(iii) methoxymethane
(iv) 2-hydroxybutanal
(v) buta-1,3-diene
(vi) 4-chloropent-2-yne
(vii) 1-bromobut-2-ene
(viii) 5-oxohexanoic acid
(ix) 3-ethyl-4-ethenylheptane
(x) 2,44-trimethylpent-2-ene
(xi) 2- methyl-1-phenylpropan-1-amine
(xii) 2,2- dimethyl-4oxopentanenitrile
(xiii) 2-ethoxypropane
(xiv) 1-fluoro-4-methyl-2-nitrobenzene
(xv) 3-bromo-2-methylpentanal
(xvi) Acetophenone
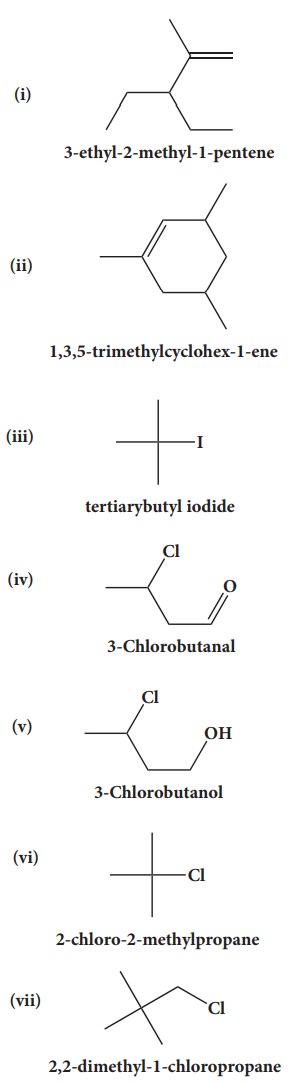
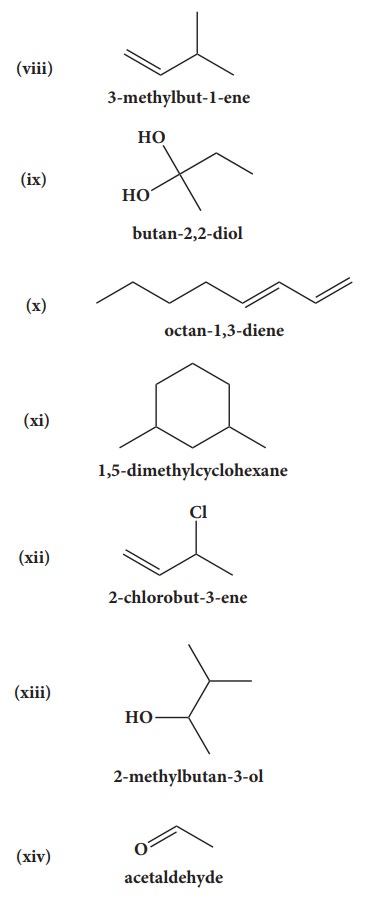
39. Give the structure for the following compound.
i. 3- ethyl - 2 methyl -1-pentene
ii. 1,3,5- Trimethyl cyclohex - 1 -ene
iii. tertiary butyl iodide
iv. 3 - Chlorobutanal
v. 3 - Chlorobutanol
vi. 2 - Chloro - 2- methyl propane
vii. 2,2-dimethyl-1-chloropropane
viii. 3 - methylbut -1- ene
ix. Butan - 2, 2 - diol
x. Octane - 1,3- diene
xi. 1,5- Dimethylcyclohexane
xii. 2-Chlorobut - 3 - ene
xiii. 2 - methylbutan - 3 - ol
xiv. acetaldehyde
Answers:
40. Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.
Na
+ C + N → NaCN
(from
organic compounds)
FeSO4
+ 2 NaOH → Fe(OH)2 + Na2SO4
6NaCN + [Fe(OH)2] → Na4
[Fe(CN)6] sod. ferro
cyanide +
2NaOH
3Na4[Fe(CN)6]
+ FeCl3 → Fe4 [Fe(CN)6]3 + 12NaCl
Prussian
blue (or) green ppt. Ferric ferro cyanide
41. Give the principle involved in the estimation of halogen in an organic compound by carius method.
Estimation
of halogens: carius method:
A
known mass of the organic compound is heated with fuming HNO3 and
AgNO3. C, H & S get oxidized to CO2, H2O
& SO2 and halogen combines with AgNO3 to form a
precipitate of silver halide.
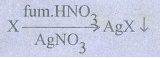
The
ppt of AgX is filtered, washed, dried and weighed. From the mass of AgX and the
mass of the organic compound taken, percentage of halogens are calculated.
42. Give a brief description of the principles of i) Fractional distillation ii) Column Chromatography
i)
Fractional distillation:
●
This is one method to purify and separate
liquids present in the mixture having their boiling point close to each other.
●
In the fractional distillation, a fractionating column is fitted with
distillation flask and a condenser.
●
A thermometer is fitted in the fractionating column near the mouth of the
condenser.
●
This will enable to record the temperature of vapour passing over the
condenser.
●
The process of separation of the components in a liquid mixture at their
respective boiling points in the form of vapours and the subsequent
condensation of those vapours is called fractional distillation.
●
The process of fractional distillation is repeated, if necessary.
●
This method finds a remarkable application in distillation of petroleum,
coal-tar and crude oil.
ii)
Column chromatography:
●
This is the simplest chromatographic method carried out in long glass cloumn
having a stop cock near the lower end.
●
This method involves separation of a mixture over a column of adsorbent
(Stationery phase) packed.
●
In the column a plug of cotton or glass wool is placed at the lower end of the
column to support the adsorbent powder.
●
The tube is uniformly packed with suitable absorbent constitute the stationary
phase. (Activatied aluminum oxides (alumina), Magnesium oxide, starch are also
used as absorbents).
●
The mixture to be separated is placed on the top of the adsorbent column.
Eluent which is a liquid or a mixture of liquids is allowed to flow down the
column slowly. Different components depending upon the degress to which the
componenets are adsorbed and complete separation takes place.
●
The most readily adsorbed substances are retained near the top and others come
down to various distances in the column.
43. Explain paper chromatography
●
Paper chromatography (PC) is an example of partition chromatography.
●
A strip of paper acts as an adsobent. This
method involves continoues differential portioning of components of a mixture
between stationary and mobile phase.
●
In paper chromatography, a special quality paper known as chromatography paper
is used. This paper act as a stationary phase.
●
A strip of chromatographic paper spotted at the base with the solution of the
mixture is suspended in a suitable solvent which act as the mobile phase.
●
The solvent rises up and flows over the spot. The paper selectively retains
different components according to their different partition in the two phases
where a chromatogram is developed.
●
The spots of the separated colored compounds are visible at different heights
from the position of initial spots on the chromatogram.
●
The spots of the separated colorless compounds may be observed either under
ultraviolent light or by the use of an appropriate spray reagent.
44. Explain varions types of constitutional isomerism (structural isomerism) in organic compounds
Constitutional
Isomers (Structural Isomerism):
This type of Isomers have same molecular formula but differ in their bonding sequence.
Structural
Isomerism
|→ a)
Chain or nuclear or skeletal isomerism
|→ b)
Position Isomerism
|→ c)
Functional Isomerism
|→ d)
Metamerism
|→ e)
Tautomerism
a)
Chain Isomerism:
Isomers
have similar molecular formula but differ in the nature of carbon skeleton (ie
straight or branched)
Eg
: CH3-CH2-CH2-CH2-CH3
n - pentane
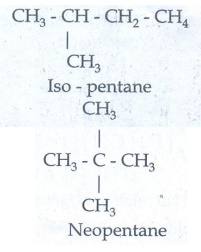
b)
Position Isomerism:
Isomers
with the same molecular formula and carbon skeleton but differ in the position
of substituent or functional group or an unsaturated linkage.
Eg.
M.FC5H10
CH3-
CH2- CH2 - CH = CH2
Pent −1 - ene
CH3-CH2-CH
= CH-CH3
Pent-2-ene
c)
Functional Isomerism:
Different
compounds with the same molecular formula but different functional groups are
said to exhibit functional isomerism.
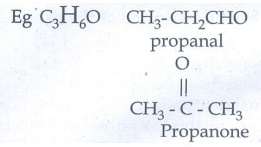
d)
Metamerism : Isomerism arising due to unequal distribution of
carbon atoms on either side of the functional group or different alkyl groups
attached to the either side of the same functional group and having same
molecular formula.
Eg
C4H10O
CH3-
O - C3H7
methyl
propyl ether
C2H5-O
- C2H5
Diethyl
ether
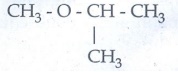
methyl
isopropyl ether
e)
Tautomerism : Single compound exists in two readily
interconvertible structures that differ in the relative position of atleast one
atomic nucleus (hydrogen)
Eg:
H
- C ≡ N ↔ H – N ≡ C
Hydrogen
cyanide ↔ Hydrogen isocyanide
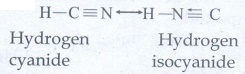
45. Describe optical isomerism with suitable example.
1)
Compound having same physical and chemical property but differ only in the
rotation of plane of the polarized light are known as optical isomer and the
phenomenon is known as optical isomerism.
2)
Some organic compounds such as glucose have the ability to rotate the plane
polarized light and they are said to be optically active compounds and this
property of a compound is called optical activity.
3)
The optical isomer, which rotates the plane of the plane polarised light to the
right or in clock wise direction is said to be dextrorotatary (dexter means
right) denoted by the sign (+).
4)
Where as the compound which rotates to the left or anticlock wise is said to be
leavo rotatory (leavues means left) denoted by sign(-).
Eg;
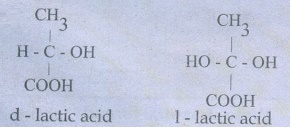
46. Briefly explain geometrical isomerism in alkene by considering 2- butene as an example.
●
Geometrical isomers which have different arrangement of groups or atoms around
a c=c. This isomeris occur due to restricted rotation of double bonds, or about
single bonds in cyclic compounds.
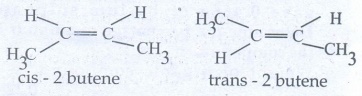
●
The cis isomer is one in which two similar groups are on the same side of the
double bond.
●
The trans isomer is that in which the two similar groups are on the opposite
side of the double bond.
47. 0.30 g of a substance gives 0.88 g of carbon dioxide and 0.54 g of water calculate the percentage of carbon and hydrogen in it.
Weight
of organic substance (w) = 0.30g
Weight
of water (x) = 0.54g
Weight
of CO2(y) = 0.88g
Percentage
of carbon = (12/44) × (y/w) × 100
=
(12/44) × (0.88/0.30) × 100
=
80%
Percentage
of Hydrogen = (12/18) × (x/w) × 100
= (2/18) × (0.54/0.30) × 100
= 20%
48. The ammonia evolved form 0.20 g of an organic compound by kjeldahl method neutralised 15ml of N/20 sulphare acid solution. Calculate the percentage of Nitrogen.
Percentage
of nitrogen = [ 1.4 × normality of acid × volume of acid used ] / [ Mass of the
compound taken ]
=
[ 1.4 × 0.05 × 15ml ] / 0.20
=
5.25%
49. 0.32 g of an organic compound, after heating with fuming nitric acid and barium nitrate crystals is a sealed tube game 0.466 g of barium sulphate. Determine the percentage of sulphur in the compound.
Weight
of organic compound (w) = 0.32g
Weight
of Barium sulphate (x) = 0.466g
Percentage
of sulphur = (32/233) × (x/w) × 100
=
(32/233) × (0.466/0.32) × 100
=
20.02%
50. 0.24g of an organic compound gave 0.287 g of silver chloride in the carius method. Calculate the percentage of chlorine in the compound.
Weight
of organic compound (w)
=
0.24g
Weight
of silver chloride (x) = 0.287g
Percentage
of chlorine =
=
(35.5 × 143.5) × (x/w) × 100
=
(35.5/143.5) × (0.287/0.24) × 100
=
29.58%
51. In the estimation of nitrogen present in an organic compound by Dumas method 0.35 g yielded 20.7 mL of nitrogen at 150 C and 760 mm pressure. Calculate the percentage of nitrogen in the compound
Percentage
of nitrogen = [ 28 / 22400 ] × [ Volume of N2 at STP / Weight of
organic compound ] × 100
= [282/22400] × [20.7/0.35] × 100 = 7.39%
Evaluate Yourself
1. Give two example for each of the following type of organic
compounds.
i) non - benzonoid aromatic
ii) aromatic heterocyclic
iii) alicyclicand
iv) aliphatic open chain
i)
non - benzonoid aromatic
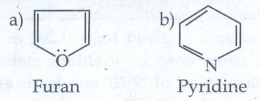
ii)
Aromatic heterocyclic
iii)
Alicyclic
a)
Cyclo propane
b)
Cyclobutane
iv)
Aliphatic open chain
a)
CH3 -CH2 - CH2 - CH3 = n - butane
b)
CH3 CH2 - CH2 - CH2 - CH3
= n - pentane
2. Write structural formula for the following compounds.
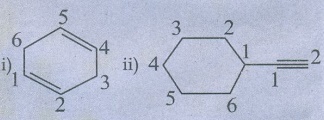
i) Cyclohexa −1, 4 - diene
ii) Ethynyl cyclohexane
Ans
: 
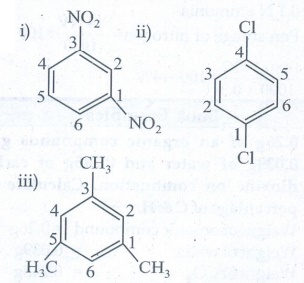
3. Write structural formula for the following compounds (March
19)
i) m -
dinitrobenzene
ii) p - dichloro
benzene
iii) 1, 3, 5 -
Trimethyl benzene
Ans
:
4. Write all the possible isomers of molecular formula C4H10O
and identify the isomerism found in them.
C4H10O
Isomers
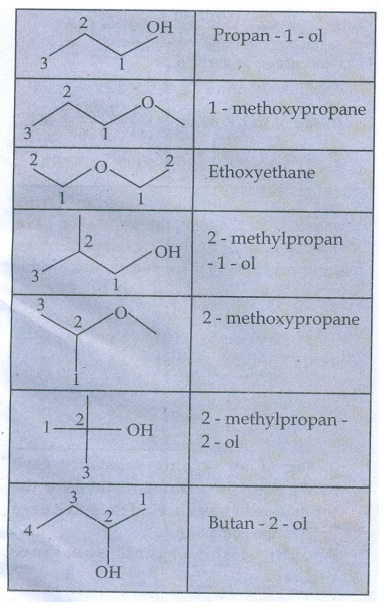
5. 0.2346g of an organic compound containing C, H & O on
combustion gives 0.2754g of H2O and 0.4488 g CO2
Calculate the % composition of C, H & O in the organic compound [C = 52.17,
H = 13.04,O = 34.79]
[C = 52.17, H = 13.04, O = 34.79]
Weight
of organic substance
(w)
= 0.2346g
Weight
of water (x) = 0.2754g
Weight
of CO2 (y) = 0.4488g
Percentage
of carbon = (12/44) × (y/w) × 100
=
(12/44) × (0.4488/0.2346) × 100 = 52.17%
Percentage
of hydrogen
=
(2/18) × (x/w) × 100
=
(2/18) × (0.2754/0.2346) × 100 = 13.04%
Percentage
of oxygen = [100 - (52.17 + 13.04)]
=
100 – 65.21
=
34.79%
6. 0.16g of an
organic compound was heated in a carius tube and H2SO4
acid formed was precipitated with BaCl2 . The mass of BaSO4
was 0.35g. Find the percentage of sulphur.
Weight
of organic substance (w) = 0.16g.
Weight
of Barium sulphate (x) = 0.35g
Percentage
of Sulphur
=
(32/233) × (x/w) × 100
=
(32/233) × (0.35/0.16) × 100 = 30.04%
7. 0.185g of an organic compound when treated with Cone. HNO3
and silver nitrate gave 0.320g of silver bromide. Calculate the % of bromine in
the compound. (Ag = 108, Br = 80)
Weight
of organic substance (w) = 0.185g
Weight
of silver bromide (x) = 0.320g
Percentage
of bromine
=
(80/188) × (x / w) × 100
=
(80/188) × (0.32/0.185) × 100 = 73.6%
8. 0.40g of an iodo substituted organic compound gave 0.235 g of
Agl by carius method. Calculate the percentage of iodine in the compound (Ag =
1081 = 127)
Weight
of organic substance
(w)
= 0.40g
Weight
of silver iodide (x) = 0.235g
Percentage
of iodine
=
(127/235) × (x/w) × 100
=
(127/235) × (0.235/0.40) × 100 = 31.75%
9. 0.33g of an organic compound containing phosphorous gave
0.397 g of Mg2P2O7 by the analysis. Calculate
the percentage of P in the compound (MFW of Mg2P2O7 is
222P = 31)
Weight
of organic substance (w) = 0.33g
Weight
of Mg2P2O7(x) = 0.397g
Percentage
of phosphorus
=
(62/222) × (x/w) × 100
=
(62/222) × (0.397/0.33) × 100 = 33.59%
10. 0.3g of an
organic compound on kjeldahl’s analysis gave enough ammonia to just neutralize
30 ml of 0.1 N H2SO4. Calculate the percentage of
nitrogen is the compound.
Weight
of organic substance(w) = 0.3g
Strength
of H2SO4 used (N) = 0.1N
Volume
of H2SO4 used (V) = 30 ml
30
ml of 0.1N H2SO4 = 30 ml of 0.1 N ammomia
Percentage
of nitrogen = [ 14 NV/1000 ] × 100
[
14 × 0.1 × 30 ] / [ 1000 × 0.3 ] × 100 = 14%
Related Topics