Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
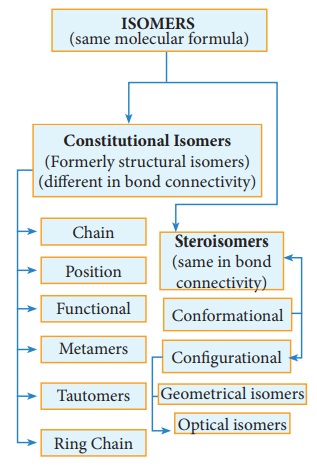
Stereoisomerism
Stereoisomerism:
The isomers which have same bond connectivity but different arrangement of groups or atoms in space are known as stereoisomers. This branch of chemistry dealing with the study of three-dimensional nature (spactial arrangement) of molecules is known as stereo chemistry. The metabolic activities in living organisms, natural synthesis and drug synthesis involve various stereoisomers.
Steroisomerism:
Geometrical isomerism:
Geometrical isomers are the stereoisomers which have different arrangement of groups or atoms around a rigid frame work of double bonds. This type of isomerism occurs due to restricted rotation of double bonds, or about single bonds in cyclic compounds.
In alkenes, the carbon-carbon double bond is sp2 hybridized. The carbon-carbon double bond consists of a σ bond and a π bond. The σ bond is formed by the head on overlap of sp2 hybrid orbitals. The π bond is formed by the side wise overlap of ‘p’ orbitals. The presence of the π bond lock the molecule in one position. Hence, rotation around C=C bond is not possible. This restriction of rotation about C-C double bond is responsible for geometrical isomerism in alkenes.
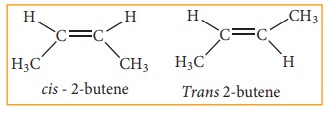
These two compounds are termed as geometrical isomers and are distinguished from each other by the terms cis and trans. The cis isomer is one in which two similar groups are on the same side of the double bond. The trans isomers is that in which the two similar groups are on the opposite side of the double bond, hence this type of isomerism is often called cis-trans isomerism.
The cis-isomer can be converted to trans isomer or vice versa is only if either isomer is heated to a high temperature or absorbs light. The heat supplies the energy (about 62kcal/ mole) to break the π bond so that rotation about σ bond becomes possible. Upon cooling, the reformation of the π bond can take place in two ways giving a mixture both cis and trans forms of trans-2-butene and cis-2-butane.
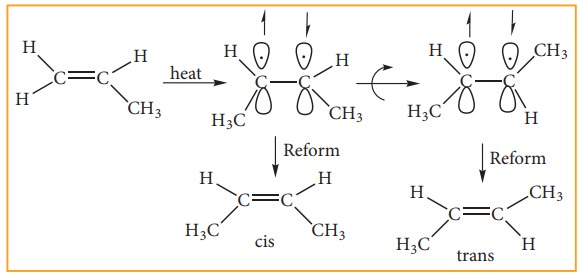
Generally the trans isomer is more stable than the corresponding cis isomers. This is because in the cis isomer, the bulky groups are on the same side of the double bond. The steric repulsion of the groups makes the cis isomers less stable than the trans isomers in which bulky groups are on the opposite side. These cis and trans isomers have different chemical property is. They can be separated by fractional distillation, gas chromatography etc., All alkenes with identical substrate do not show geometrical isomerism. Geometrical isomerism is possible only when each double bonded C atom is attached to two different atoms or groups eg. In propene no geometrical isomers are possible because one of the double bonded carbon has two identical H atoms.
Cis-trans isomerism is also seen around single bond. For eg: 1,3-butadiene has two double bonds in conjugation. CH2=CH-CH=CH2. It can exist in infinite number of conformations, but the following two extreme conformations are important.
ii) Oximes and azo compounds:
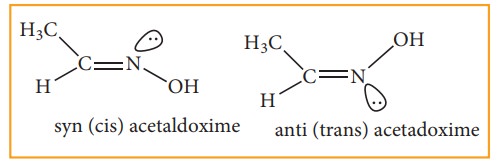
Restricted rotation around C=N (oximes) gives rise to geometrical isomerism in oximes. Here ‘syn’ and ‘anti’ are used instead of cis and trans respectively. In the syn isomer the H atom of a doubly bonded carbon and –OH group of doubly bonded nitrogen lie on the same side of the double bond, while in the anti isomer, they lie on the opposite side of the double bond. For eg:
Optical Isomerism
Compounds having same physical and chemical property but differ only in the rotation of plane of the polarized light are known as optical isomers and the phenomenon is known as optical isomerism.
Some organic compounds such as glucose have the ability to rotate the plane of the plane polarized light and they are said to be optically active compounds and this property of a compound is called optical activity. The optical isomer, which rotates the plane of the plane polarised light to the right or in clockwise direction is said to be dextrorotary (dexter means right) denoted by the sign (+), whereas the compound which rotates to the left or anticlockwise is said to be leavo rotatory (leavues means left) denoted by sign(-). Dextrorotatory compounds are represented as ‘d’ or by sign (+) and lavorotatory compounds are represented as ‘l’ or by sign (-).
Enantiomerism and optical activity
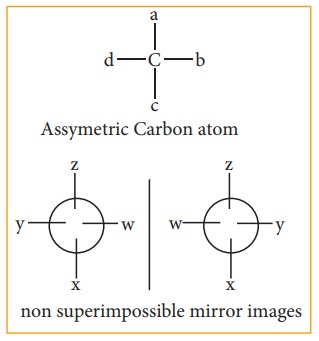
An optically active substance may exist in two or more isomeric forms which have same physical and chemical properties but differ in terms of direction of rotation of plane polarized light, such optical isomers which rotate the plane of polarized light with equal angle but in opposite direction are known as enantiomers and the phenom-enon is known as enantiomerism. Isomers which are non-super impossible mirror im-ages of each other are called enantiomers.
Conditions for enantiomerism or optical isomerism
A carbon atom whose tetra valency is satisfied by four different substituents (atoms or groups) is called asymmetric carbon or chiral carbon. It is indicated by an asterisk as C*. A molecule possessing chiral carbon atom and non-super impossible to its own mirror image is said to be a chiral molecule or asymmetric, and the property is called chirality or dissymmetry.
Related Topics