Multiple choice questions - Choose the best answer: Fundamentals of Organic Chemistry | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Choose the best answer: Fundamentals of Organic Chemistry
Fundamentals of Organic Chemistry
Choose the best answer
1. Select the molecule which has only one π bond.
a) CH3– CH = CH – CH3
b) CH3– CH = CH – CHO
c) CH3– CH = CH – COOH
d) All of these
2. In the hydrocarbon
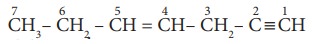
state of hybridisation of carbon 1,2,3,4 and 7 are in the following sequence.
a) sp, sp, sp3, sp2, sp3
b) sp2, sp, sp3, sp2, sp3
c) sp, sp, sp2, sp, sp3
d) none of these
3. The general formula for alkadiene is
a) CnH2n
b) CnH2n-1
c) CnH2n-2
d) CnHn-2
4. Structure of the compound whose IUPAC name is 5,6 - dimethylhept - 2 - ene is
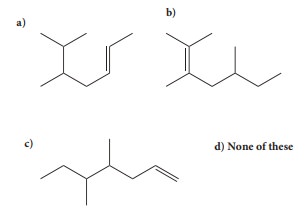
Ans: a
5. The IUPAC name of the Compound is
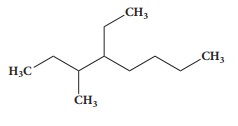
a) 2,3 - Diemethylheptane
b) 3- Methyl -4- ethyloctane
c) 5-ethyl -6-methyloctane
d) 4-Ethyl -3 - methyloctane.
6. Which one of the following names does not fit a real name?
a) 3 – Methyl –3–hexanone
b) 4–Methyl –3– hexanone
c) 3– Methyl –3– hexanol
d) 2– Methyl cyclo hexanone.
7. The IUPAC name of the compound CH3–CH= CH – C ≡ CH is
a) Pent - 4 - yn-2-ene
b) Pent -3-en-l-yne
c) pent – 2– en – 4 – yne
d) Pent – 1 – yn –3 –ene
8. IUPAC name of
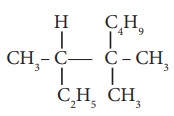 is
is
a) 3,4,4 – Trimethylheptane
b) 2 – Ethyl –3, 3– dimethyl heptane
c) 3, 4,4 – Trimethyloctane
d) 2 – Butyl -2 –methyl – 3 – ethyl-butane.
9. The IUPAC name of
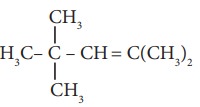 is
is
a) 2,4,4 – Trimethylpent -2-ene
b) 2,4,4 – Trimethylpent -3-ene
c) 2,2,4 – Trimethylpent -3-ene
d) 2,2,4 – Trimethylpent -2-ene
10. The IUPAC name of the compound
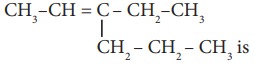
a) 3 – Ethyl -2– hexene
b) 3 – Propyl -3– hexene
c) 4 – Ethyl – 4 – hexene
d) 3 – Propyl -2-hexene
11. The IUPAC name of the compound
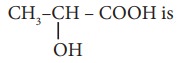
a) 2 – Hydroxypropionic acid
b) 2 – Hydroxy Propanoic acid
c) Propan – 2– ol –1 – oic acid
d) 1 – Carboxyethanol.
12. The IUPAC name of
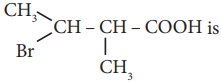
a) 2 – Bromo -3 – methyl butanoic acid
b) 2 - methyl - 3- bromobutanoic acid
c) 3 - Bromo - 2 - methylbutanoic acid
d) 3 - Bromo - 2, 3 - dimethyl propanoic acid.
13. The structure of isobutyl group in an organ-ic compound is
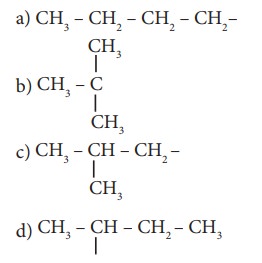
Ans: c
14. The number of stereoisomers of 1, 2 – dihy-droxy cyclopentane
a) 1
b)2
c) 3
d) 4
15. Which of the following is optically active?
a) 3 – Chloropentane
b) 2 Chloro propane
c) Meso – tartaric acid
d) Glucose
16. The isomer of ethanol is
a) acetaldehyde
b) dimethylether
c) acetone
d) methyl carbinol
17. How many cyclic and acyclic isomers are possible for the molecular formula C3H6O?
a) 4
b) 5
c) 9
d) 10
18. Which one of the following shows functional isomerism?
a) ethylene
b) Propane
c) ethanol
d) CH2Cl2
19. 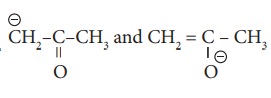
a) resonating structure
b) tautomers
c) Optical isomers
d) Conformers.
20. Nitrogen detection in an organic compound is carried out by Lassaigne’s test. The blue colour formed is due to the formation of.
a) Fe3[Fe(CN)6]2
b) Fe4[Fe(CN)6]3
c) Fe4[Fe(CN)6]2
d) Fe3 [Fe(CN)6]3
21. Lassaigne’s test for the detection of nitrogen fails in
a) H2N – CO– NH.NH2.HCl
b) NH2 – NH2. HCl
c) C6H5 – NH – NH2. HCl
d) C6H5 CONH2
22. Connect pair of compounds which give blue colouration / precipitate and white precipi-tate respectively, when their Lassaigne’s test is separately done.
a) NH2 NH2 HCl and ClCH2–CHO
b) NH2 CS NH2 and CH3 – CH2Cl
c) NH2 CH2 COOH and NH2 CONH2
d) C6H5NH2 and ClCH2 – CHO.
23. Sodium nitropruside reacts with sulphide ion to give a purple colour due to the for-mation of
a) [Fe(CN)5 NO]3-
b) [Fe(NO)5 CN]+
c) [Fe(CN)5NOS]4-
d) [Fe (CN)5 NOS]3-
24. An organic Compound weighing 0.15g gave on carius estimation, 0.12g of silver bromide. The percentage of bromine in the Compound will be close to
a) 46%
b) 34%
c) 3.4%
d) 4.6%
25. A sample of 0.5g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50mL of 0.5M H2SO4. The remaining acid after neutralisation by ammonia consumed 80mL of 0.5 MNaOH, The percentage of nitrogen in the organic compound is.
a) 14%
b) 28%
c) 42%
d) 56%
26. In an organic compound, phosphorus is estimated as
a) Mg2P2O7
b) Mg3(PO4)2
c) H3PO4
d) P2O5
27. Ortho and para-nitro phenol can be separated by
a) azeotropic distillation
b) destructive distillation
c) steam distillation
d) cannot be separated
28. The purity of an organic compound is determined by
a) Chromatography
b) Crystallisation
c) melting or boiling point
d) both (a) and (c)
29. A liquid which decomposes at its boiling point can be purified by
a) distillation at atmospheric pressure
b) distillation under reduced pressure
c) fractional distillation
d) steam distillation.
30. Assertion:
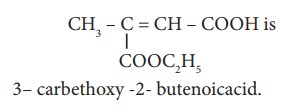
Reason: The principal functional group gets lowest number followed by double bond (or) triple bond.
a) both the assertion and reason are true and the reason is the correct explanation of assertion.
b) both assertion and reason are true and the reason is not the correct explanation of assertion.
c) assertion is true but reason is false
d) both the assertion and reason are false.
Related Topics