Equation, Procedure, Calculation, Example - Estimation of halogens | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Estimation of halogens
Estimation of halogens:
carius method:
A known mass of the organic compound is heated with fuming HNO3
and AgNO3. C,H &S get oxidized to CO2, H2O
& SO2 and halogen combines with AgNO3 to form a
precipitate of silver halide.
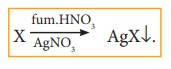
The ppt of AgX is filtered, washed, dried and weighed. From the mass of AgX and the mass of the organic compound taken, percentage of halogens are calculated.
A known mass of the substance is taken along with fuming HNO3
and AgNO3 is taken in a clean carius tube. The open end of the
Carius tube is sealed and placed in a iron tube for 5 hours in the range at
530-540 k Then the tube is allowed to cool and a small hole is made in the tube
to allow gases produced to escape. The tube is broken and the ppt is filtered,
washed, dried and weighed. From the mass of AgX obtained, percentage of halogen
in the organic compound is calculated.
Weight
of the organic compound: w g
Weight
of AgCl precipitate = a g
143.5
g of AgCl contains 35.5 g of Cl
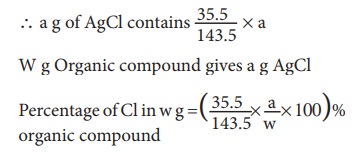
Let
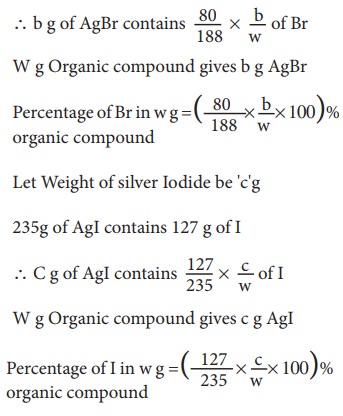
Weight of silver Bromide be 'b'g
188g
of AgBr contains 80 g of Br
EXAMPLE :
0.284 g of an organic
substance gave 0.287 g AgCl in a carius method for the
estimation of halogen. Find the Percentage of Cl in the compound.
Weight
of the organic substance = 0.284 g
Weight
of AgCl is = 0.287 g
143.5
g of AgCl contains 35.5 g of chlorine

Related Topics