Equation, Procedure, Calculation, Example - Kjeldahls method - Estimation of nitrogen | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Kjeldahls method - Estimation of nitrogen
There are two methods for the estimation of nitrogen in an organic compound. They are 1. Dumas method 2. Kjeldahls method
Kjeldahls method:
This method is carried much more easily than the Dumas method. It is used largely in the analysis of foods and fertilizers. Kjeldahls method is based on the fact that when an organic compound containing nitrogen is heated with Conc. H2SO4, the nitrogen in it is quantitatively converted to ammonium sulphate. The resultant liquid is then treated with excess of alkali and then liberated ammonia gas absorbed in excess of standard acid. The amount of ammonia (and hence nitrogen) is determined by finding the amount of acid neutralized by back titration with same standard alkali.
Procedure:
A weighed quantity of the substance (0.3 to 0.5 g) is placed in a special long – necked Kjeldahl flask made of pyrex glass. About 25 mL of Conc. H2SO4 together with a little K2SO4 and CuSO4 (catalyst) are added to it the flask is loosely stoppered by a glass bulb and heated gently in an inclined position. The heating is continued till the brown color of the liquid first produced, disappears leaving the contents clear as before. At this point all the nitrogen in the substance is converted to (NH4)2SO4. The Kjeldahl flask is then cooled and its contents are diluted with same distilled water and then carefully transferred into a 1 lit round bottom flask. An excess NaOH solution is poured down the side of the flask and it is fitted with a Kjeldahl trap and a water condenser. The lower end of the condenser dips in a measured volume of excess the N/20 H2SO4 solution. The liquid in the round bottom flask is then heated and the liberated ammonia is distilled into sulphuric acid. The Kjeldahl trap serves to retain any alkali splashed up on vigorous boiling. (Refer Fig. 11.3)
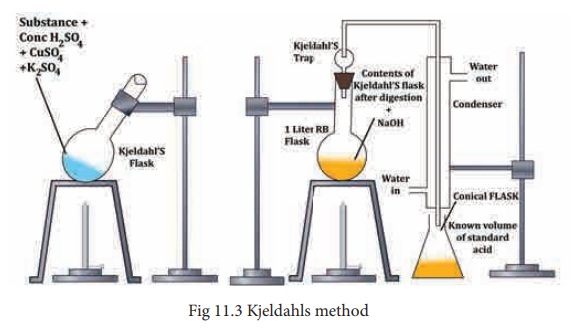
When no more ammonia passes over (test the distillate with red litmus) the receiver is removed. The excess of acid is then determined by titration with alkali, using phenolphthalein as the indicator.
Calculation:
Weight of the substance = Wg.
Volume of H2SO4 required for the complete neutralisation of evolved NH3 = V mL. Strength of H2SO4 used to neutralise NH3 = N
Let the Volume and the strength of NH3 formed are V1 and N1 respectively
we know that V1N1 = VN
The amount of nitrogen present in the w g
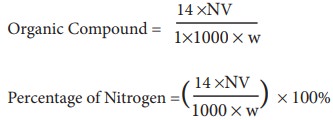
Example :
0.6 g of an organic compound was Kjeldalised and NH3 evolved was absorbed into 50 mL of semi-normal solution of H2SO4. The residual acid solution was diluted with distilled water and the volume made up to 150 mL. 20 mL of this diluted solution required 35 mL of N/20 NaOH solution for complete neutralization.
Calculate the % of N in the compound.
Weight of Organic compound = 0.6g
Volume of sulphuric acid taken = 50mL
Strength of sulphuric acid taken= 0.5 N
20 ml of diluted solution of unreacted sulphuric acid was neutralised by 35 mL of 0.05 N Sodium hydroxide
Strength of the diluted sulphuric acid = 35 × 0.05 / 20
= 0.0875 N
Volume of the sulphuric acid remaining after reaction with = V1 mL
Organic compound Strength of H2SO4 = 0.5N
Volume of the diluted H2SO4 = 150 mL
Strength of the diluted sulphuric acid = 0.0875 N
V1 = 150 ×0.087 / 0.5
= 26.25 mL
Volume of H2SO4 consumed by ammonia = 50 - 26.25 = 23.75 mL
23.75 mL of 0.5 N H2SO4 ≡ 23.75mL of 0.5N NH3
The amount of Nitrogen present in the 0.6 = 14g / [1000 mL × 1 N] × 23.75 × 0.5N = 0.166g
Percentage of Nitrogen = [0.166/0.6] × 100 = 27.66 %
Related Topics