Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Isomerism in organic compounds and its types
Isomerism
in organic compounds:
The
term ‘isomerism’ was given by Berzelius, and its represents of existence of two
or more compounds with the same molecular formula but different structure and
properties (physical, chemical, or both). Compounds exhibiting this isomerism
are called isomers. The difference in properties of two isomers is due to
difference in (bond connectivity or spatial arrangement) the arrangement of
atoms within their molecules. Isomerism is broadly divided into two types. i.
Constitutional isomerism, ii. stereoisomerism.
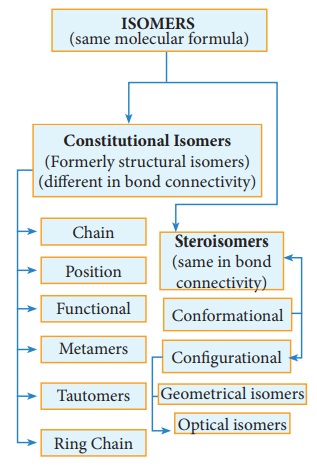
Constitutional isomers (Formerly structural isomers):
This
type of isomers have same molecular formula but differ in their bonding
sequence. Structural or constitutional isomerism is further classified into
following types.
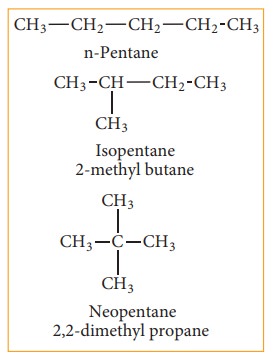
(a) Chain or nuclear or skeletal isomerism:
These
isomers differ in the way in which the carbon atoms are bonded to each other in
a carbon chain or in other words isomers have similar molecular formula but
differ in the nature of the carbon skeleton (ie. Straight or branched)
(b) Position isomerism:
If
different compounds belonging to same homologous series with the same molecular
formula and carbon skeleton, but differ in the position of substituent or
functional group or an unsaturated linkage are said to exhibit position
isomerism.
Example:
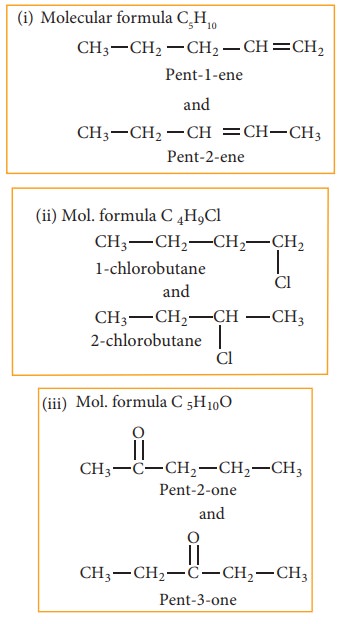
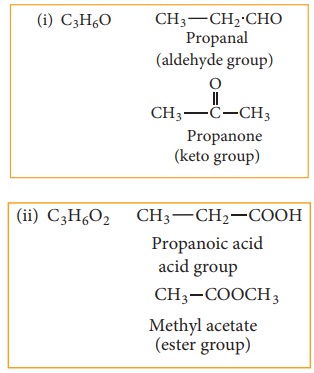
(c) Functional isomerism:
Different
compounds having same molecular formula but different functional groups are
said to exhibit functional isomerism.
Example:
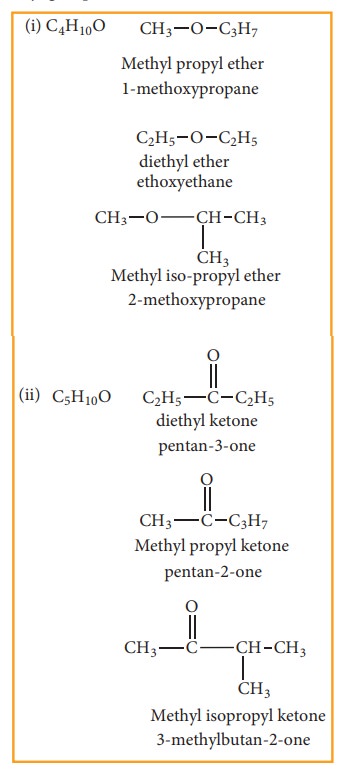
(d) Metamerism:
This type of isomerism is a special kind of
structural isomerism arises due to the unequal distribution of carbon atoms on
either side of the functional group or different alkyl groups attached to the
either side of the same functional group and having same molecular formula.
This isomerism is shown by compounds having functional group such as ethers,
ketones, esters and secondary amines between two alkyl groups.
(e) Tautomerism:
It is a special type of functional isomerism
in which a single compound exists in two readily inter con-vertible structures
that differ markedly in the relative position of atleast one atomic nucleus,
generally hydrogen. The two dif-ferent structures are known as tautomers. There
are several types of tautomerism and the two important types are dyad and triad
systems.
(i) Dyad system:
In this system hydrogen atom oscillates between
two directly linked polyvalent atoms. Eg:
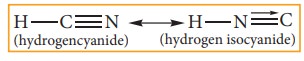
In
this example hydrogen atom oscillates between carbon & nitrogen atom
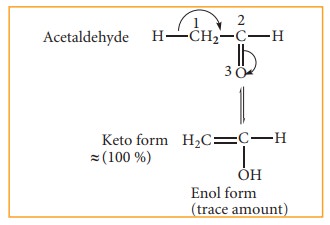
(ii) Triad system: In this system hydrogen atom oscillates between three polyvalent atoms. It involves 1,3 migration of hydrogen atom from one polyvalent atom to other within the mole-cule. The most important type of triad system is keto–enol tautomerism and the two groups of tautomers are ketoform and enol-form. The polyvalent atoms involved are one oxygen and two carbon atoms. Enolisation is a process in which keto-form is converted to enol form. Both tautomeric forms are not equally stable. The less stable form is known as labile form
Example:
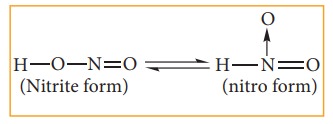
Nitro-aci tautomerism.
(f) Ring chain isomerism:
In this type of isomerism,
compounds having same molecular formula but differ in terms of bonding of
carbon atom to form open chain and cyclic structures for eg:
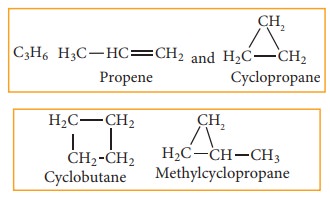
Stereoisomerism:
The
isomers which have same bond connectivity but different arrangement of groups
or atoms in space are known as stereoisomers. This branch of chemistry dealing
with the study of three-dimensional nature (spactial arrangement) of molecules
is known as stereo chemistry. The metabolic activities in living organisms,
natural synthesis and drug synthesis involve various stereoisomers.
Steroisomerism:
Geometrical isomerism:
Geometrical
isomers are the stereoisomers which have different arrangement of groups or
atoms around a rigid frame work of double bonds. This type of isomerism occurs
due to restricted rotation of double bonds, or about single bonds in cyclic
compounds.
In
alkenes, the carbon-carbon double bond is sp2 hybridized. The
carbon-carbon double bond consists of a σ bond and a π bond. The σ bond is
formed by the head on overlap of sp2 hybrid orbitals. The π bond is
formed by the side wise overlap of ‘p’ orbitals. The presence of the π bond
lock the molecule in one position. Hence, rotation around C=C bond is not
possible. This restriction of rotation about C-C double bond is responsible for
geometrical isomerism in alkenes.
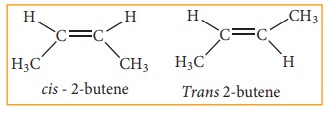
These
two compounds are termed as geometrical isomers and are distinguished from each
other by the terms cis and trans. The cis isomer is one in which two similar
groups are on the same side of the double bond. The trans isomers is that in
which the two similar groups are on the opposite side of the double bond, hence
this type of isomerism is often called cis-trans isomerism.
The
cis-isomer can be converted to trans isomer or vice versa is only if either
isomer is heated to a high temperature or absorbs light. The heat supplies the
energy (about 62kcal/ mole) to break the π bond so that rotation about σ bond
becomes possible. Upon cooling, the reformation of the π bond can take place in
two ways giving a mixture both cis and trans forms of trans-2-butene and
cis-2-butane.
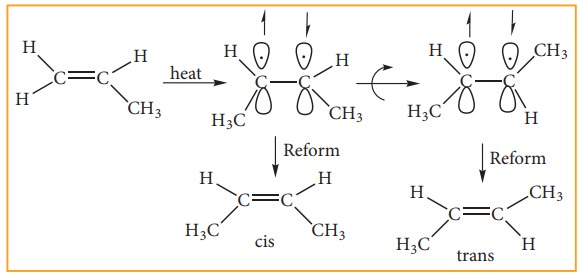
Generally
the trans isomer is more stable than the corresponding cis isomers. This is because in the cis isomer, the bulky groups
are on the same side of the double bond. The steric repulsion of the groups
makes the cis isomers less stable than the trans isomers in which bulky groups
are on the opposite side. These cis and trans isomers have different chemical
property is. They can be separated by fractional distillation, gas
chromatography etc., All alkenes with identical substrate do not show
geometrical isomerism. Geometrical isomerism is possible only when each double
bonded C atom is attached to two different atoms or groups eg. In propene no
geometrical isomers are possible because one of the double bonded carbon has
two identical H atoms.
Cis-trans
isomerism is also seen around single bond. For eg: 1,3-butadiene has two double
bonds in conjugation. CH2=CH-CH=CH2. It can exist in
infinite number of conformations, but the following two extreme conformations
are important.
ii) Oximes and azo compounds:
Restricted
rotation around C=N (oximes) gives rise to geometrical isomerism in oximes.
Here ‘syn’ and ‘anti’ are used instead of cis and trans respectively. In the
syn isomer the H atom of a doubly bonded carbon and –OH group of doubly bonded
nitrogen lie on the same side of the double bond, while in the anti isomer,
they lie on the opposite side of the double bond. For eg:
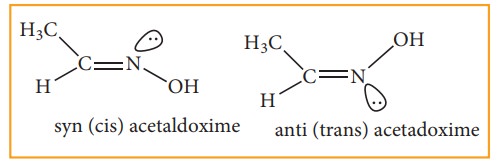
Optical Isomerism
Compounds
having same physical and chemical property but differ only in the rotation of
plane of the polarized light are known as optical isomers and the phenomenon is
known as optical isomerism.
Some
organic compounds such as glucose have the ability to rotate the plane of the
plane polarized light and they are said to be optically active compounds and
this property of a compound is called optical activity. The optical isomer,
which rotates the plane of the plane polarised light to the right or in
clockwise direction is said to be dextrorotary (dexter means right) denoted by
the sign (+), whereas the compound which rotates to the left or anticlockwise
is said to be leavo rotatory (leavues means left) denoted by sign(-).
Dextrorotatory compounds are represented as ‘d’ or by sign (+) and lavorotatory
compounds are represented as ‘l’ or by sign (-).
Enantiomerism and optical activity
An
optically active substance may exist in two or more isomeric forms which have
same physical and chemical properties but differ in terms of direction of
rotation of plane polarized light, such optical isomers which rotate the plane
of polarized light with equal angle but in opposite direction are known as
enantiomers and the phenom-enon is known as enantiomerism. Isomers which are
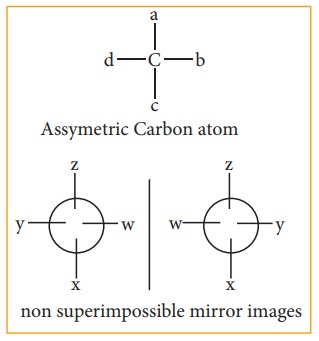
non-super impossible mirror im-ages of each other are called enantiomers.
Conditions for enantiomerism or optical isomerism
A
carbon atom whose tetra valency is satisfied by four different substituents
(atoms or groups) is called asymmetric carbon or chiral carbon. It is indicated
by an asterisk as C*. A molecule possessing chiral carbon atom and non-super
impossible to its own mirror image is said to be a chiral molecule or
asymmetric, and the property is called chirality or dissymmetry.
Related Topics