Equation, Procedure, Calculation, Example - Estimation of sulphur | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Estimation of sulphur
Estimation of sulphur:
Carius method:
A known mass of the
organic substance is heated strongly with fuming HNO3.
C & H get oxidized to CO2& H2O while sulphur is
oxidized to sulphuric acid as per the following reaction.
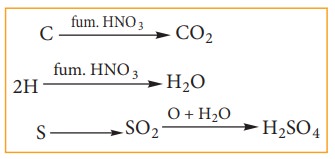
The
resulting solution is treated with excess of BaCl2 solution H2SO4
present in the solution in quantitatively converted into BaSO4, from
the mass of BaSO4, the mass of sulphur and hence the percentage of
sulphur in the compound can be calculated.
Procedure:
A
known mass of the organic compound is taken in clean carius tube and added a
few mL of fuming HNO3. The tube is the sealed. It is then placed in
an iron tube and heated for about 5 hours. The tube is allowed to cool to
temperature and a small hole is made to allow gases produced inside to escape.
The carius tube is broken and the content collected in a beaker. Excess of BaCl2
is added to the beaker H2SO4 acid formed as a result of
the reaction is converted to BaSO4. The precipitate of BaSO4
is filtered, washed, dried and weighed. From the mass of BaSO4,
percentage of S is found.
Mass
of the organic compound = w g
Mass
of the BaSO4 formed = x g
233g
of BaSO4 contains 32 g of Sulphur

Example -2
In
an estimation of sulphur by carius method, 0.2175 g of the substance gave 0.5825
g of BaSO4 calculate the percentage composition of S in the
compound.
Weight
of organic compound 0.2175 g
Weight
of BaSO4 0.5825 g
233
g of BaSO4 contains 32 g of S

Related Topics