Equation, Procedure, Calculation, Example - Dumas method - Estimation of nitrogen | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Dumas method - Estimation of nitrogen
There are two methods for the estimation of nitrogen in an organic compound. They are 1. Dumas method 2. Kjeldahls method
Dumas method:
This method is based upon the fact that nitrogenous compound when heated with cupric oxide in an atmosphere of CO2 yields free nitrogen. Thus
CX HY NZ + (2x + Y/2) CuO → x CO2 + Y/2 H2O + z/2 N2 + (2x + Y/2) Cu
Traces of oxide of nitrogen, which may be formed in some cases, are reduced to elemental nitrogen by passing over heated copper spiral.
The apparatus used in Dumas method consists of CO2 generator, combustion tube, Schiffs nitrometer. (Fig. 11.4)
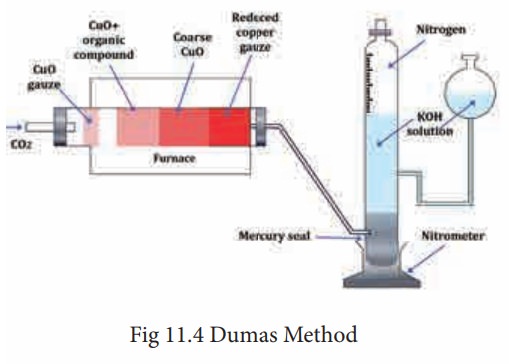
CO2 generator:
CO2 needed in this process is prepared by heating magnetite or sodium bicarbonate contained in a hard glass tube or by the action of dil. HCl on marble in a kipps apparatus. The gas is passed through the combustion tube after being dried by bubbling through Conc. H2SO4.
Combustion Tube:
The combustion tube is heated in a furnace is charged with a) A roll of oxidized copper gauze to prevent the back diffusion of the products of combustion and to heat the organic substance mixed with CuO by radiation b) a weighed amount of the organic substance mixed with excess of CuO, C) a layer of course CuO packed in about 2/3 of the entire length of the tube and kept in position by loose asbestos plug on either side; this oxidizes the organic vapors passing through it, and d) a reduced copper spiral which reduces any oxides of nitrogen formed during combustion to nitrogen.
Schiff’s nitro meter:
The nitrogen gas obtained by the decomposition of the substance in the combustion tube is mixed with considerable excess of CO2 It is estimated by passing nitrometer when CO2 is absorbed by KOH and the nitrogen gets collected in the upper part of graduated tube.
Procedure:
To start with the tap of nitro meter is left open. CO2 is passed through the combustion tube to expel the air in it. When the gas bubbles rising through, the potash solution fails to reach the top of it and is completely absorbed it shows that only CO2 is coming and that all air has been expelled from the combustion tube. The nitrometer is then filled with KOH solution by lowering the reservoir and the tap is closed. The combustion tube is now heated in the furnace and the temperature rises gradually. The nitrogen set free form the compound collects in the nitro meter. When the combustion is complete a strong current of CO2 is sent through, the apparatus in order to sweep the last trace of nitrogen from it. The volume of the gas gets collected is noted after adjusting the reservoir so that the solution in it and the graduated tube is the same. The atmospheric pressure and the temperature are also recorded.
Calculations:
Weight of the substance taken = wg
Volume of nitrogen = V1 L
Room Temperature = T1 K
Atmospheric Pressure = P mm of Hg
Agueen tension at
room temperature = P1 mm of Hg
Pressure of dry nitrogen = (P-P1) = P1 mm of Hg.
Let p0 V0 and T0 be the pressure, Volume and temperature respectively of dry nutrogen at STP,
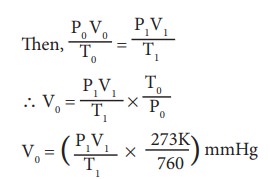
Calculation of percentage of nitrogen. 22.4 L of N2 at STP weigh 28gof N2
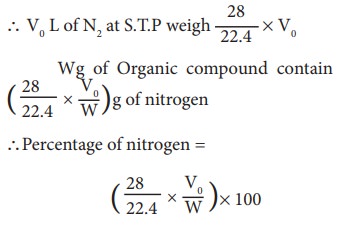
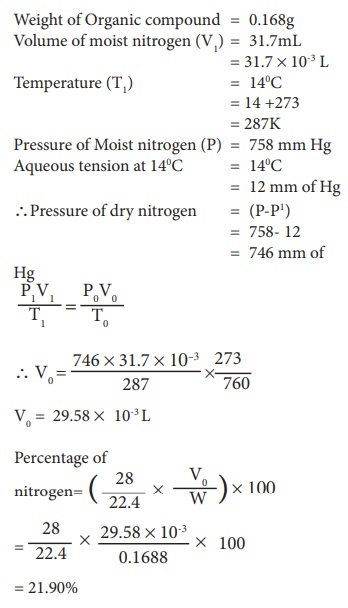
Example Problem:
0.1688 g when analyzed by the Dumas method yield 31.7 mL of moist nitrogen measured at 14º C and 758 mm mercury pressure. Determine the % of N in the substance (Aqueous tension at 14 º C =12 mm)
Related Topics