Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Rutherford's α - particle scattering experiment
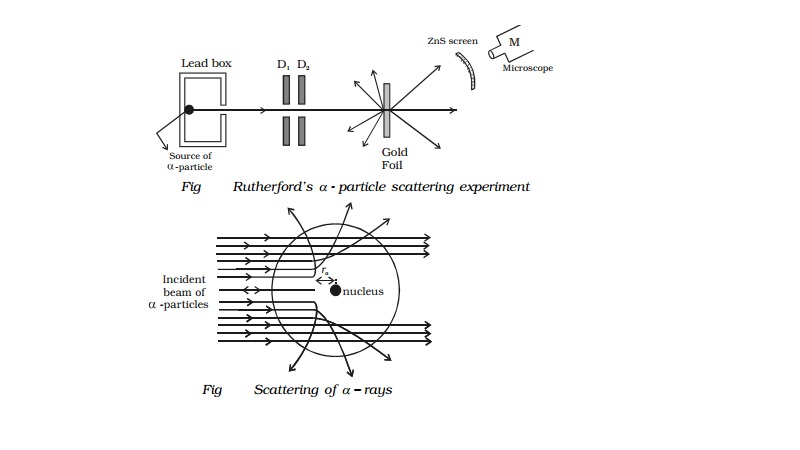
Rutherford's
α - particle scattering experiment
Rutherford and his associates studied the
scattering of the α - particles by a thin gold foil in order to
investigate the structure of the atom. An α-particle is a positively charged particle
having a mass equal to that of helium atom and positive charge in magnitude
equal to twice the charge of an electron. They are emitted by many radioactive
elements. The scattering of α-particles provide useful information about the
structure of the atom.
Experimental arrangement
A fine pencil of α−particles was obtained from a radioactive
material like radium or radon by placing it in a lead box with narrow opening
as shown in Fig.
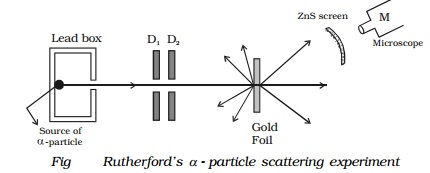
The α-particles are emitted from the source in all
possible directions, but only a narrow beam emerges from the lead box. The
remaining α-particles are absorbed in the lead box itself. After passing
through the diaphragms D1 and D2, a narrow beam of α-particles incident on a thin gold foil, are scattered through
different angles. The scattered α-particles strike a fluorescent screen coated
with zinc sulphide. When the α-particles strike the screen, tiny flashes of
light are produced. The observations can be made with the help of a low power
microscope.
Observations and conclusions
(i)
Most of the α
particles either passed straight through
the gold foil or were scattered by only
small angles of the
order of
a few degrees.
This observation led to the conclusion that an atom has a lot of empty space as
shown in Fig.
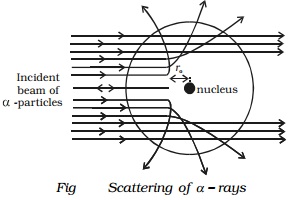
(ii) A few α particles were scattered in the backward
direction, which led Rutherford to conclude that the whole of the positive
charge was concentrated in a tiny space of about 10-14m. This region
of the atom was named as nucleus. Only a small number of particles approaches
the nucleus of the atom and they were deflected at large angles.
Distance of closest approach
An α particle directed towards the centre of the
nucleus will move close upto a distance ro
as shown in Fig, where its kinetic energy will appear as electrostatic
potential energy. After this, the α particle begins to retrace its path. This
distance ro is known as
the distance of the closest approach.
Let m
and v be the mass and velocity of the
α particle directed towards the centre of the nucleus. Then, the
kinetic energy of the particle,
Ek = ½ mv2 …………… (1)
Since, charge of an α-particle is 2e and that of the nucleus of the atom is Ze, the
electrostatic potential energy of the α particle, when at a distance ro from the centre of the
nucleus is given by,
Ep
= ( 1/ 4πε0 ) . (2e )( Ze)
/ r0 ............(2)
where Z is the atomic number of the atom and εo, the
permittivity of free space.
On reaching the distance of the closest
approach ro, the kinetic
energy of the α particle appears as its potential energy.
Ep
= Ek
( 1/ 4πε0 ) . (2e )( Ze)
/ r0 = ½ mv2
r0 = ( 1/ 4πε0 ) 4Ze2 / mv2
Related Topics