Chapter: Basic & Clinical Pharmacology : Antiseizure Drugs
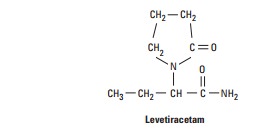
Levetiracetam
LEVETIRACETAM
Levetiracetam
is a piracetam analog that is ineffective against sei-zures induced by maximum
electroshock or pentylenetetrazol but has prominent activity in the kindling
model. This is the first major drug with this unusual preclinical profile that
is effective against partial seizures.
Mechanism of Action
Levetiracetam
binds selectively to the synaptic vesicular protein SV2A. The
function of this protein is not understood but it is likely that levetiracetam
modifies the synaptic release of glutamate and GABA through an action on
vesicular function.
Clinical Uses
Levetiracetam
is marketed for the adjunctive treatment of partial seizures in adults and
children for primary generalized tonic-clonic seizures and for the myoclonic
seizures of juvenile myoclonic epi-lepsy. Adult dosing can begin with 500 or
1000 mg/d. The dosage can be increased every 2–4 weeks by 1000 mg to a maximum
dosage of 3000 mg/d. The drug is dosed twice daily. Adverse effects include
somnolence, asthenia, ataxia, and dizziness. Less common but more serious are
mood and behavioral changes; psychotic reactions are rare. Drug interactions
are minimal; levetiracetam is not metabo-lized by cytochrome P450. Oral
formulations include extended-release tablets; an intravenous preparation is
also available.
Pharmacokinetics
Oral
absorption of levetiracetam is nearly complete; it is rapid and unaffected by
food, with peak plasma concentrations in 1.3 hours. Kinetics are linear.
Protein binding is less than 10%. The plasma half-life is 6–8 hours, but may be
longer in the elderly. Two thirds of the drug is excreted unchanged in the
urine; the drug has no known active metabolites.
Related Topics