Chapter: Basic & Clinical Pharmacology : Antiseizure Drugs
Basic Pharmacology of Antiseizure Drugs
BASIC PHARMACOLOGY OF ANTISEIZURE
DRUGS
Chemistry
Until
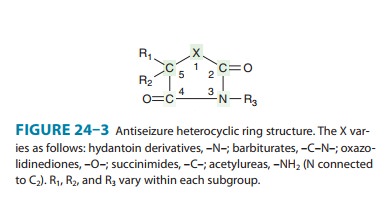
1990, approximately 16 antiseizure drugs were available, and 13 of them can be
classified into five very similar chemical groups: barbiturates, hydantoins,
oxazolidinediones, succinim-ides, and acetylureas. These groups have in common
a similar heterocyclic ring structure with a variety of substituents (Figure
24–3). For drugs with this basic structure, the substitu-ents on the
heterocyclic ring determine the pharmacologic class, either anti-MES or
antipentylenetetrazol. Very small changes in structure can dramatically alter
the mechanism of action and clinical properties of the compound. The remaining
drugs in this older group—carbamazepine, valproic acid, and the
benzo-diazepines—are structurally dissimilar, as are the newer com-pounds
marketed since 1990, ie, eslicarbazepine, felbamate, gabapentin, lacosamide,
lamotrigine, levetiracetam, oxcarba-zepine, pregabalin, retigabine, rufinamide,
stiripentol, tiagabine, topiramate, vigabatrin, and zonisamide.
Pharmacokinetics
The antiseizure drugs exhibit many similar pharmacokinetic properties—even those whose structural and chemical properties are quite diverse—because most have been selected for oral activ-ity and all must enter the central nervous system. Although many of these compounds are only slightly soluble, absorption is usually good, with 80–100% of the dose reaching the circulation. Most antiseizure drugs (other than phenytoin, tiagabine, and valproic acid) are not highly bound to plasma proteins.Antiseizure drugs are cleared chiefly by hepatic mechanisms, although they have low extraction ratios . Many are converted to active metabolites that are also cleared by the liver.
These drugs are predominantly distributed into total body water. Plasma clearance is relatively slow; many antiseizure drugs are therefore considered to be medium to long acting. Some have half-lives longer than 12 hours. Many of the older antiseizure drugs are potent inducers of hepatic microsomal enzyme activity. Compliance is better with less frequent admin-istration; thus extended-release formulations permitting once-or twice-daily administration may offer an advantage.
Related Topics