Chapter: Basic & Clinical Pharmacology : Antiseizure Drugs
Ethosuximide
DRUGS USED IN GENERALIZED
SEIZURES
ETHOSUXIMIDE
Ethosuximide
was introduced in 1960 as the third of three marketed succinimides in the USA.
Ethosuximide has very little activity against maximal electroshock but
considerable efficacy against pen-tylenetetrazol seizures; it was introduced as
a “pure petit mal” drug.
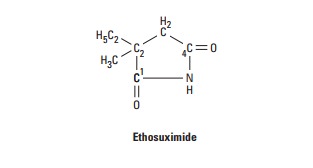
Chemistry
Ethosuximide
is the last antiseizure drug to be marketed whose origin is in the cyclic
ureide structure. The three antiseizure succinimides marketed in the USA are
ethosuximide, phensuximide, and methsuximide. Methsuximide and phensuximide
have phenyl substituents, whereas ethosuximide is 2-ethyl-2-methylsuccinimide.
Mechanism of Action
Ethosuximide
has an important effect on Ca2+ currents, reducing the low-threshold
(T-type) current. This effect is seen at therapeuti-cally relevant
concentrations in thalamic neurons. The T-type Ca2+ currents are
thought to provide a pacemaker current in thalamic neurons responsible for
generating the rhythmic cortical discharge of an absence attack. Inhibition of
this current could therefore account for the specific therapeutic action of
ethosuximide. A recently described effect on inwardly rectifying K+
channels may also be significant.
Clinical Uses
As
predicted from its activity in laboratory models, ethosuximide is particularly
effective against absence seizures, but has a very narrow spectrum of clinical
activity. Documentation of its effectiveness in human absence seizures was
achieved with long-term electroencephalographic recording techniques. Data
continue to show that ethosuximide and valproate are the drugs of choice for
absence seizures and are more effective than lamotrigine.
Pharmacokinetics
Absorption
is complete following administration of the oral dosage forms. Peak levels are
observed 3–7 hours after oral administration of the capsules. Ethosuximide is
not protein-bound. The drug is completely metabolized, principally by
hydroxylation, to inactive metabolites. Ethosuximide has a very low total body
clearance (0.25 L/kg/d). This corresponds to a half-life of approximately 40
hours, although values from 18 to 72 hours have been reported.
Therapeutic Levels & Dosage
Therapeutic
levels of 60–100 mcg/mL can be achieved in adults with dosages of 750–1500
mg/d, although lower or higher dos-ages and blood levels (up to 125 mcg/mL) may
be necessary and tolerated in some patients. Ethosuximide has a linear
relation-ship between dose and steady-state plasma levels. The drug might be
administered as a single daily dose were it not for its adverse
gastrointestinal effects; twice-a-day dosage is common.
Drug Interactions & Toxicity
Administration
of ethosuximide with valproic acid results in a decrease in ethosuximide
clearance and higher steady-state con-centrations owing to inhibition of
metabolism. No other impor-tant drug interactions have been reported for the
succinimides. The most common dose-related adverse effect of ethosuximide is
gastric distress, including pain, nausea, and vomiting. When an adverse effect
does occur, temporary dosage reductions may allow adaptation. Other
dose-related adverse effects are transient leth-argy or fatigue and, much less
commonly, headache, dizziness, hiccup, and euphoria. Behavioral changes are
usually in the direc-tion of improvement. Non–dose-related or idiosyncratic
adverse effects of ethosuximide are extremely uncommon.
Related Topics