Chapter: Basic & Clinical Pharmacology : Antiseizure Drugs
Carbamazepine
CARBAMAZEPINE
Closely
related to imipramine and other antidepressants, carbam-azepine is a tricyclic
compound effective in treatment of bipolar depression. It was initially
marketed for the treatment of trigemi-nal neuralgia but has proved useful for
epilepsy as well.
Chemistry
Although
not obvious from a two-dimensional representation of its structure,
carbamazepine has many similarities to phenytoin. The ureide moiety (–N–CO–NH2)
in the heterocyclic ring of most antiseizure drugs is also present in
carbamazepine. Three-dimensional structural studies indicate that its spatial
conforma-tion is similar to that of phenytoin.
Mechanism of Action
The
mechanism of action of carbamazepine appears to be similar to that of
phenytoin. Like phenytoin, carbamazepine shows activity against maximal
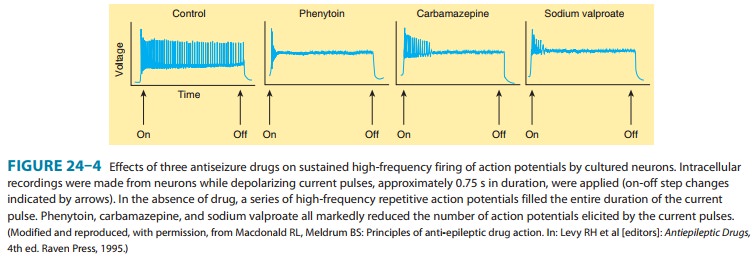
electroshock seizures. Carbamazepine, like phenytoin, blocks Na+
channels at therapeutic concentrations and inhibits high-frequency repetitive
firing in neurons in culture (Figure 24–4). It also acts presynaptically to
decrease synaptic transmission. Potentiation of a voltage-gated K+
current has also been described. These effects probably account for the
anticonvul-sant action of carbamazepine. Binding studies show that
carbam-azepine interacts with adenosine receptors, but the functional
significance of this observation is not known.
Clinical Uses
Although
carbamazepine has long been considered a drug of choice for both partial
seizures and generalized tonic-clonic seizures, some of the newer antiseizure
drugs are beginning to displace it from this role. Carbamazepine is not
sedative in its usual therapeutic range. The drug is also very effective in
some patients with trigeminal neuralgia, although older patients may tolerate
higher doses poorly, with ataxia and unsteadiness. Carbamazepine is also useful
for controlling mania in some patients with bipolar disorder.
Pharmacokinetics
The
rate of absorption of carbamazepine varies widely among patients, although
almost complete absorption apparently occurs in all. Peak levels are usually
achieved 6–8 hours after administra-tion. Slowing absorption by giving the drug
after meals helps the patient tolerate larger total daily doses.
Distribution
is slow, and the volume of distribution is roughly 1 L/kg. The drug is
approximately 70% bound to plasma proteins; no displacement of other drugs from
protein binding sites has been observed.
Carbamazepine
has a very low systemic clearance of approxi-mately 1 L/kg/d at the start of
therapy. The drug has a notable ability to induce microsomal enzymes.
Typically, the half-life of 36 hours observed in subjects after an initial
single dose decreases to as little as 8–12 hours in subjects receiving
continuous therapy. Considerable dosage adjustments are thus to be expected
during the first weeks of therapy. Carbamazepine also alters the clearance of
other drugs .
Carbamazepine
is completely metabolized in humans to several derivatives. One of these,
carbamazepine-10,11-epoxide, has been shown to have anticonvulsant activity.
The contribution of this and other metabolites to the clinical activity of
carbamazepine is unknown.
Therapeutic Levels & Dosage
Carbamazepine
is available only in oral form. The drug is effective in children, in whom a
dosage of 15–25 mg/kg/d is appropriate. In adults, daily doses of 1 g or even 2
g are tolerated. Higher dos-age is achieved by giving multiple divided doses
daily. Extended-release preparations permit twice-daily dosing for most
patients. In patients in whom the blood is drawn just before the morning dose
(trough level), the therapeutic level is usually 4–8 mcg/mL. Although many
patients complain of diplopia at drug levels above 7 mcg/mL, others can
tolerate levels above 10 mcg/mL, especially with monotherapy. Extended-release
formulations that overcome some of these issues are now available.
Drug Interactions
Drug
interactions involving carbamazepine are almost exclu-sively related to the
drug’s enzyme-inducing properties. As noted previously, the increased metabolic
capacity of the hepatic enzymes may cause a reduction in steady-state
carbamazepine concentrations and an increased rate of metabolism of other drugs,
eg, primidone, phenytoin, ethosuximide, valproic acid, and clonazepam. Other
drugs such as valproic acid may inhibit carbamazepine clearance and increase
steady-state carbam-azepine blood levels. Other anticonvulsants, however, such
as phenytoin and phenobarbital, may decrease steady-state con-centrations of
carbamazepine through enzyme induction. No clinically significant
protein-binding interactions have been reported.
Toxicity
The
most common dose-related adverse effects of carbamazepine are diplopia and
ataxia. The diplopia often occurs first and may last less than an hour during a
particular time of day. Rearrangement of the divided daily dose can often
remedy this complaint. Other dose-related complaints include mild
gastrointestinal upsets, unsteadiness, and, at much higher doses, drowsiness.
Hyponatremia and water intoxication have occasionally occurred and may be dose
related.Considerable concern exists regarding the occurrence of idio-syncratic
blood dyscrasias with carbamazepine, including fatal cases of aplastic anemia
and agranulocytosis. Most of these have been in elderly patients with
trigeminal neuralgia, and most have occurred within the first 4 months of
treatment. The mild and persistent leukopenia seen in some patients is not
necessarily an indication to stop treatment but requires careful monitoring.
The most common idiosyncratic reaction is an erythematous skin rash; other
responses such as hepatic dysfunction are unusual.
Related Topics