Chapter: Basic & Clinical Pharmacology : Antiseizure Drugs
Drug Development for Epilepsy - Antiseizure Drugs
Drug Development for Epilepsy
For
a long time it was assumed that a single antiepileptic
drug(AED) could be developed for the treatment of all forms of epi-lepsy.
However, the causes of epilepsy are extremely diverse, encompassing genetic and
developmental defects and infective, traumatic, neoplastic, and degenerative
disease processes. Drug therapy to date shows little evidence of etiologic
specificity. There is some specificity according to seizure type (Table 24–1),
which is most clearly seen with generalized seizures of the absence type. These
are typically seen with 2–3 Hz spike-and-wave
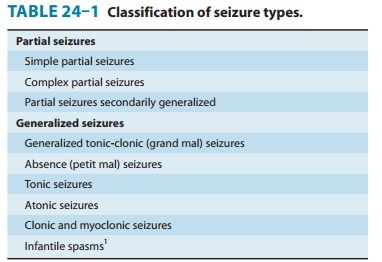
discharges
on the electroencephalogram, which respond to etho-suximide and valproate but
can be exacerbated by phenytoin and carbamazepine. Drugs acting selectively on
absence seizures can be identified by animal screens, using either threshold
pentyle-netetrazol clonic seizures in mice or rats or mutant mice showing
absence-like episodes (so-called lethargic, star-gazer, or tottering mutants).
In contrast, the maximal electroshock
(MES) test, with suppression of the tonic extensor phase, identifies drugs
such as phenytoin, carbamazepine, and lamotrigine, which are active against
generalized tonic-clonic seizures and complex par-tial seizures. The maximal
electroshock test as the major initial screen for new drugs has led
predominantly to the identification of drugs with a mechanism of action
involving prolonged inac-tivation of the voltage-gated Na+ channel.
Limbic seizures induced in rats by the process of electrical kindling (involving
repeated episodes of focal electrical stimulation) probably pro-vide a better
screen for predicting efficacy in complex partial seizures.
Existing
antiseizure drugs provide adequate seizure control in about two thirds of
patients. So-called “drug resistance” may be observed from the onset of
attempted therapy or may develop after a period of relatively successful
therapy. Explanations are being sought in terms of impaired access of the drugs
to target sites or insensitivity of target molecules to them. In children, some
severe seizure syndromes associated with pro-gressive brain damage are very
difficult to treat. In adults, some focal seizures are refractory to
medications. Some, particularly in the temporal lobe, are amenable to surgical
resection. Some of the drug-resistant population may respond to vagus nervestimulation (VNS), a
nonpharmacologic treatment for epilepsynow widely approved for treatment of
patients with partial sei-zures. VNS is indicated for refractory cases or for
patients in whom antiseizure drugs are poorly tolerated. Stimulating
elec-trodes are implanted on the left vagus nerve, and the pacemaker is
implanted in the chest wall or axilla. Use of this device may permit seizure
control with lower doses of drugs. Other devices,using various paradigms of
electrical stimulation, are in clinical development.
New
antiseizure drugs are being sought not only by the screen-ing tests noted above
but also by more focused approaches. Compounds are sought that act by one of
three mechanisms: (1) enhancement of GABAergic (inhibitory) transmission, (2)
diminution of excitatory (usually glutamatergic) transmission, ormodification
of ionic conductances. Presynaptic effects ontransmitter release appear
particularly important, and some molecular targets are known, eg, SV2A
.
Although
it is widely recognized that current antiseizure drugs are palliative rather
than curative, successful strategies for identify-ing drugs that are either
disease modifying or that prevent epilep-togenesis have proved elusive. Neuronal
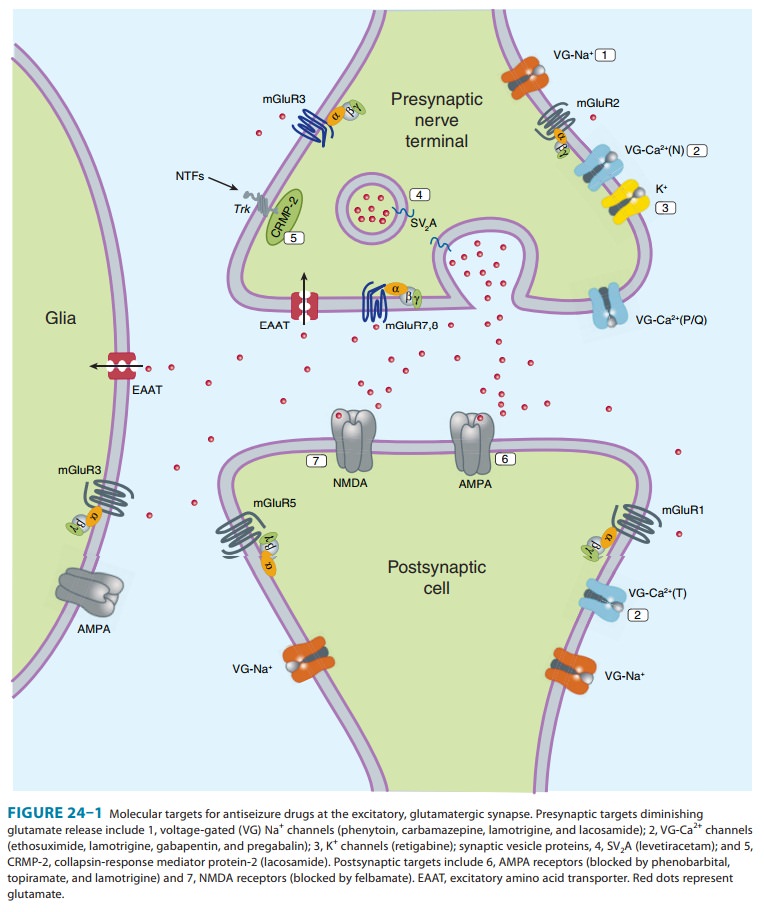
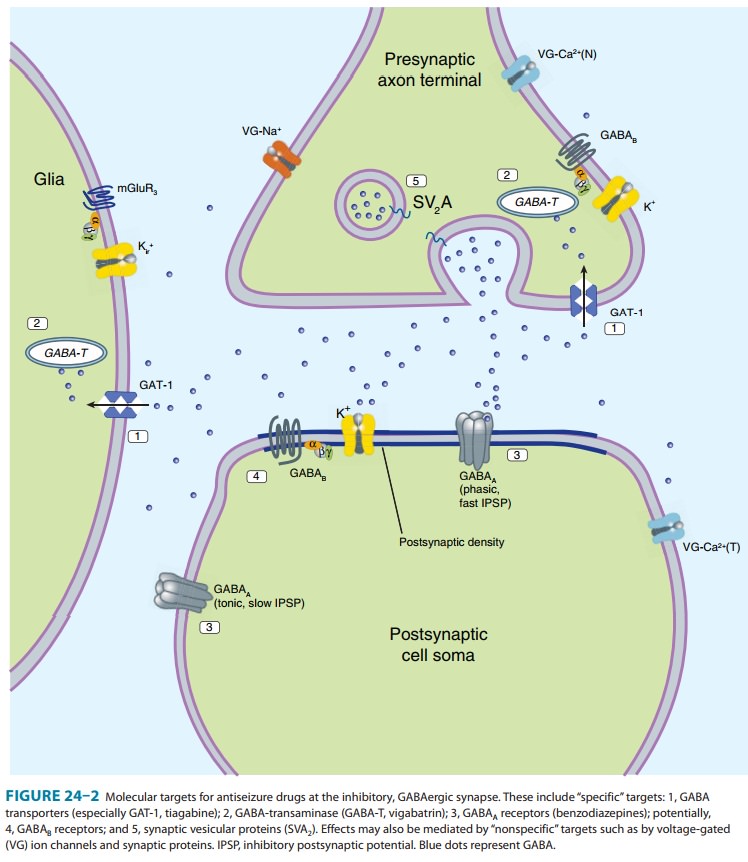
targets for current and potential antiseizure drugs include both excitatory and
inhibitory synapses. Figure 24–1 represents a glutamatergic (excitatory)
synapse, and Figure 24–2 indicates targets in a GABAergic (inhibitory) synapse.
Related Topics