Chapter: Basic & Clinical Pharmacology : Antihypertensive Agents
Hypertension & Regulation of Blood Pressure
HYPERTENSION & REGULATION OF
BLOOD PRESSURE
Diagnosis
The
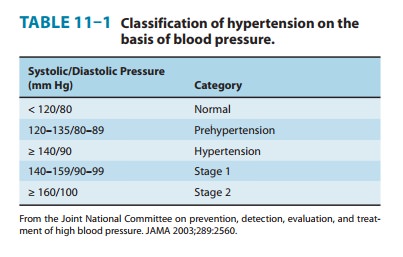
diagnosis of hypertension is based on repeated, reproducible measurements of
elevated blood pressure (Table 11–1). The diagnosis serves primarily as a
prediction of consequences for the patient; it seldom includes a statement
about the cause of hypertension.Epidemiologic studies indicate that the risks
of damage to kidney, heart, and brain are directly related to the extent of
blood pressure elevation. Even mild hypertension (blood pressure 140/90 mm Hg) increases the risk of eventual
end-organ damage. Starting at 115/75 mm Hg, cardiovascular disease risk doubles
with each increment of 20/10 mm Hg throughout the blood pres-sure range. Both
systolic hypertension and diastolic hypertension are associated with end-organ
damage; so-called isolated systolic hypertension is not benign. The risks—and
therefore the urgency of instituting therapy—increase in proportion to the magnitude
of blood pressure elevation. The risk of end-organ damage at any level of blood
pressure or age is greater in African Americans and relatively less in
premenopausal women than in men. Other posi-tive risk factors include smoking;
metabolic syndrome, including obesity, dyslipidemia, and diabetes;
manifestations of end-organ damage at the time of diagnosis; and a family
history of cardiovas-cular disease.
It
should be noted that the diagnosis of hypertension depends on measurement of
blood pressure and not on symptoms reported by the patient. In fact,
hypertension is usually asymptomatic until overt end-organ damage is imminent
or has already occurred.
Etiology of Hypertension
A
specific cause of hypertension can be established in only 10–15% of patients.
Patients in whom no specific cause of hyper-tension can be found are said to
have essential or primary hyper-tension. Patients with a
specific etiology are said to have
secondary hypertension. It is important to consider specific causes in
eachcase, however, because some of them are amenable to definitive surgical
treatment: renal artery constriction, coarctation of the aorta,
pheochromocytoma, Cushing’s disease, and primary aldosteronism.
In
most cases, elevated blood pressure is associated with an over-all increase in
resistance to flow of blood through arterioles, whereas cardiac output is
usually normal. Meticulous investigation of auto-nomic nervous system function,
baroreceptor reflexes, the renin-angiotensin-aldosterone system, and the kidney
has failed to identify a single abnormality as the cause of increased
peripheral vascular resistance in essential hypertension. It appears,
therefore, that ele-vated blood pressure is usually caused by a combination of
several (multifactorial) abnormalities. Epidemiologic evidence points to
genetic factors, psychological stress, and environmental and dietary factors
(increased salt and decreased potassium or calcium intake) as contributing to
the development of hypertension. Increase in blood pressure with aging does not
occur in populations with low daily sodium intake. Patients with labile
hypertension appear more likely than normal controls to have blood pressure
elevations after salt loading.
The
heritability of essential hypertension is estimated to be about 30%. Mutations
in several genes have been linked to various rare causes of hypertension.
Functional variations of the genes for angiotensinogen, angiotensin-converting
enzyme (ACE), the β2 adrenoceptor, and α adducin (a cytoskeletal protein) appear to
contribute to some cases of essential hypertension.
Normal Regulation of Blood Pressure
According
to the hydraulic equation, arterial blood pressure (BP) is directly
proportionate to the product of the blood flow (cardiac output, CO) and the
resistance to passage of blood through pre-capillary arterioles (peripheral
vascular resistance, PVR):
BP = CO × PVR
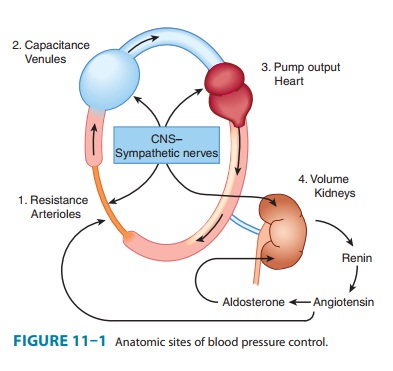
Physiologically,
in both normal and hypertensive individuals, blood pressure is maintained by
moment-to-moment regulation of cardiac output and peripheral vascular
resistance, exerted at three anatomic sites (Figure 11–1): arterioles,
postcapillary venules (capacitance vessels), and heart. A fourth anatomic
control site, the kidney, contributes to maintenance of blood pressure by
regu-lating the volume of intravascular fluid. Baroreflexes, mediated by
autonomic nerves, act in combination with humoral mechanisms, including the
renin-angiotensin-aldosterone system, to coordinate function at these four control
sites and to maintain normal blood pressure. Finally, local release of
vasoactive substances from vascu-lar endothelium may also be involved in the
regulation of vascular resistance. For example, endothelin-1 constricts and nitric oxide dilates blood vessels.
Blood
pressure in a hypertensive patient is controlled by the same mechanisms that
are operative in normotensive subjects. Regulation of blood pressure in
hypertensive patients differs from healthy patients in that the baroreceptors
and the renal blood volume-pressure control systems appear to be “set” at a
higher level of blood pressure. All antihypertensive drugs act by interfer-ing
with these normal mechanisms, which are reviewed below.
A. Postural Baroreflex
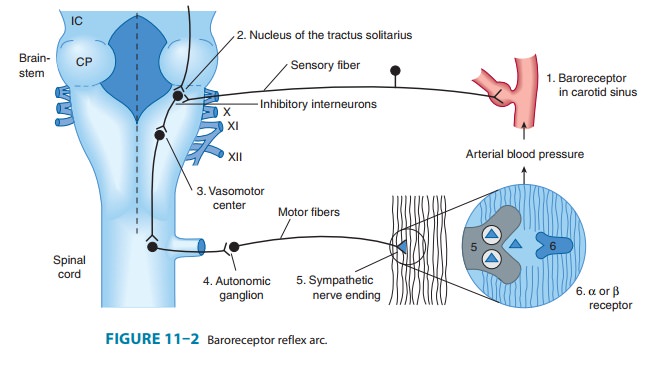
Baroreflexes
are responsible for rapid, moment-to-moment adjust-ments in blood pressure,
such as in transition from a reclining to an upright posture (Figure 11–2).
Central sympathetic neurons arising from the vasomotor area of the medulla are
tonically active. Carotid baroreceptors are stimulated by the stretch of the
vessel walls brought about by the internal pressure (arterial blood pres-sure).
Baroreceptor activation inhibits central sympathetic dis-charge. Conversely,
reduction in stretch results in a reduction in baroreceptor activity. Thus, in
the case of a transition to upright posture, baroreceptors sense the reduction
in arterial pressure that results from pooling of blood in the veins below the
level of the heart as reduced wall stretch, and sympathetic discharge is
disin-hibited. The reflex increase in sympathetic outflow acts through nerve
endings to increase peripheral vascular resistance (constric-tion of
arterioles) and cardiac output (direct stimulation of the heart and
constriction of capacitance vessels, which increases venous return to the
heart), thereby restoring normal blood pres-sure. The same baroreflex acts in
response to any event that lowers arterial pressure, including a primary
reduction in peripheral vas-cular resistance (eg, caused by a vasodilating
agent) or a reduction in intravascular volume (eg, due to hemorrhage or to loss
of salt and water via the kidney).
B. Renal Response to Decreased Blood Pressure
By
controlling blood volume, the kidney is primarily responsible for long-term
blood pressure control. A reduction in renal perfu-sion pressure causes
intrarenal redistribution of blood flow and increased reabsorption of salt and
water. In addition, decreased pressure in renal arterioles as well as
sympathetic neural activity (via β adrenoceptors) stimulates production of
renin, which increases production of angiotensin II. Angiotensin II causes (1)
direct constriction of resis-tance vessels and (2) stimulation of aldosterone
synthesis in the adrenal cortex, which increases renal sodium absorption and
intra-vascular blood volume. Vasopressin released from the posterior pituitary
gland also plays a role in maintenance of blood pressure through its ability to
regulate water reabsorption by the kidney.
Related Topics