Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Detection of elements(carbon and hydrogen) in organic compounds
Detection
of elements in organic compounds
Introduction
The
first step in the analysis of an organic compound is the detection of elements
present in it. The principal elements are carbon, hydrogen and oxygen In
addition to these they may contain nitrogen, sulphur and halogens. Phosphorous.
Metals like Li, Mg, Zn are present in certain organometalic compounds.
Detection of carbon and hydrogen
If
the compound under investigation is or-ganic, there is no need to test for
carbon. This test is performed only to establish whether a given compound is
organic or not. With the exception of few compounds like CCl4, CS2
all organic compounds also contain hydro-gen. The presence of both these
elements is confirmed by the following common test.
(i) Copper oxide test:
The organic substance is mixed with about
three times its weight of dry copper oxide by grinding. The mixture is then
placed in a hard glass test tube fitted with a bent delivery tube. The other
end of which is dipping into lime water in an another test tube. The mixture is
heated strongly and the following reaction take place.
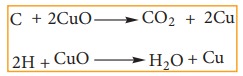
Thus
if carbon is present, it is oxidized to CO2 which turns lime water
milky. If hydrogen is also present, it will be oxidized to water which
condenses in small droplets on the cooler wall of the test tube and inside the
bulb. Water is collected on anhydrous CuSO4 which turns anhydrous
CuSO4 blue. This confirms the presence of C and H in the compound.
Detection
of nitrogen by lassaigne sodium fusion test: This is a good test for the
detection of nitrogen in all classes of nitrogenous compound and it involves
the preparation of sodium fusion extract
This
method involves the conversion of covalently bonded N, S or halogen present in
the organic compounds to corresponding water soluble ions in the form of sodium
salts For this purpose a small piece of Na dried by pressing between the folds
of a filter paper is taken in a fusion tube and it is gently heated.
When
it melts to a shining globule, put a pinch of the organic compound on it. Heat
the tube till reaction ceases and becomes red hot. Plunge it in about 50 mL of
distilled water taken in a china dish and break the bottom of the tube by
striking against the dish. Boil the contents of the dish for about 10 mts and
filter. This filtrate is known as lassaignes extract or sodium fusion extract
and it used for detection of nitrogen, sulfur and halogens present in organic
compounds.
(ii) Test for Nitrogen:
If nitrogen is present it gets
converted to sodium cyanide which reacts with freshly prepared ferrous sulphate
and ferric ion followed by conc. HCl and gives a Prussian blue color or green
color or precipitate. It confirms the presence of nitrogen. HCl is added to
dissolve the greenish precipitate of ferrous hydroxide produced by the excess
of NaOH on FeSO4 which would otherwise mark the Prussian blue precipitate.
The following reaction takes part in the formation of Prussian blue.
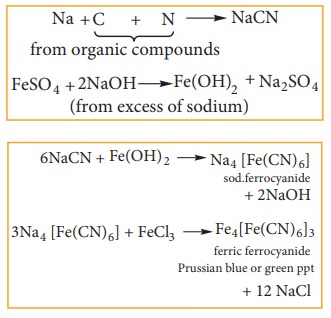
Incase
if both N & S are present, a blood red color is obtained due to the
following reactions.
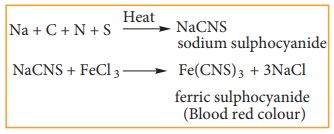
iii) Test for sulphur:
a)
To a portion of the lassaigne's extract, add freshly prepared sodium nitro
prusside solution. A deep violet or purple colouration is obtained. This test
is also used to detect S2- in inorganic salt analysis
Na2S+Na2
[Fe (CN5) NO]→Na4 [Fe (CN5) NOS]
sodium
nitro prusside
b)
Acidify another portion of lassaigne's extract with acetic acid and add lead
acetate solution. A black precipitate is obtained.
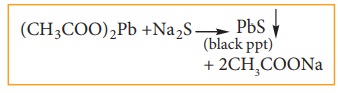
c) Oxidation test: The organic substances are fused with a mixture of KNO3 and Na2CO3.
The sulphur, if present is oxidized to sulphate.
Na2CO3 + S +3O → Na2SO4
+ CO2
The
fused mass is extracted with water, acidified with HCl and then BaCl2
solution is added to it. A white precipitate indicates the presence of sulphur.
BaCl2 + Na2SO4 → BaSO4 +
2NaCl
iv) Test for halogens:
To
another portion of the lassaigne’s filtrate add dil HNO3 warm gently
and add AgNO3 solution.
a)
Appearance of curdy white precipitate soluble in ammonia solution indicates the
presence of chlorine.
b)
Appearance of pale yellow precipitate sparingly soluble in ammonia solution
indicates the presence of bromine.
c)
Appearance of a yellow precipitate insoluble in ammonia solution indicates the
presence of iodine.
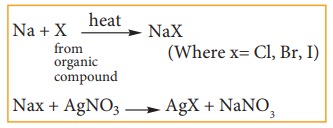
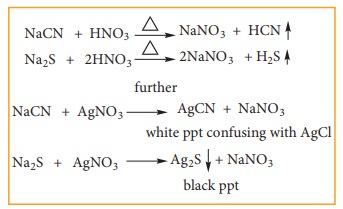
If
N or S is present in the compound along with the halogen, we might obtain NaCN
and Na2S in the solution, which interfere with the detection of the
halogen in the AgNO3 test Therefore we boil the lassaignes extract
with HNO3 which decomposes NaCN and Na2S as
v) Test for phosphorous:
A
solid compound is strongly heated with a mixture of Na2CO3
& KNO3. phosphorous present in the compound is oxidized to
sodium phosphate. The residue is extracted with water and boiled with Conc. HNO3.
A solution of ammonium molybdate is added to the above solution. A canary
yellow coloration or precipitate shows the presence of phosphorous.
Related Topics