Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Cardiovascular Disease
Congenital Heart Disease
Congenital Heart Disease
Preoperative Considerations
Congenital heart disease encompasses a
seemingly endless list of abnormalities that may be detected in infancy, early
childhood, or, less commonly, adult-hood. The incidence of congenital heart
disease in all live births approaches 1%. The natural history of some defects
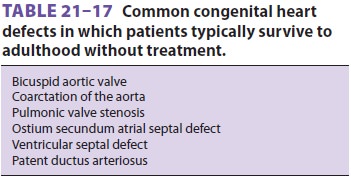
is such that patients often survive to adulthood ( Table 21–17). Moreover, the
number of surviving adults with congenital heart disease is steadily
increasing, possibly as a result of advances in surgical and medical treatment.
An increasing number of patients with congenital heart disease may therefore be
encountered during noncardiac surgery and obstetric deliveries. Knowledge of
the
anatomy of the original heart structure
defect and of any corrective repairs is essential prior to anesthetiz-ing the
patient with congental heart disease (CHD).
The complex nature and varying
pathophysiol-ogy of congenital heart defects make classification difficult. A
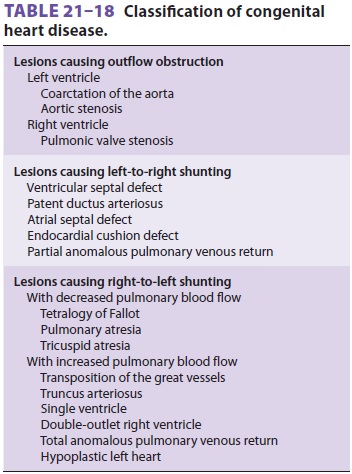
commonly used scheme is presented in Table 21–18. Most patients present with
cyano-sis, congestive heart failure, or an asymptomatic abnormality. Cyanosis
is typically the result of an abnormal intracardiac communication that allows
unoxygenated blood to reach the systemic arterial circulation (right-to-left
shunting). Congestive heart failure is most prominent with defects that either
obstruct left ventricular outflow or mark-edly increase pulmonary blood flow.
The latter is usually due to an abnormal intracardiac commu-nication that
returns oxygenated blood to the right heart (left-to-right shunting). Whereas
right-to-left shunts generally decrease pulmonary blood flow, some complex
lesions increase pulmonary blood flow—even in the presence of right-to-left
shunt-ing. In many cases, more than one lesion is present.
In fact, survival (prior to surgical
correction) with some anomalies (eg, transposition, total anoma-lous venous
return, pulmonary atresia) depends on the simultaneous presence of another
shunting lesion (eg, patent ductus arteriosus, patent foramen ovale,
ventricular septal defect). Chronic hypox-emia in patients with cyanotic heart
disease typi-cally results in erythrocytosis. This increase in red cell mass,
which is due to enhanced erythropoietin secretion from the kidneys, serves to
restore tissue oxygen concentration to normal. Unfortunately, blood viscosity
can also rise to the point at which it may interfere with oxygen delivery. When
tissue oxygenation is restored to normal, the hematocrit is stable (usually <65%), and symptoms of the hyper-viscosity syndrome
are absent, the patient is said to have compensated erythrocytosis. Patients
with uncompensated erythrocytosis do not establish this equilibrium; they have
symptoms of hypervis-cosity and may be at risk of thrombotic complica-tions,
particularly stroke. The last is aggravated by dehydration. Children younger
than age 4 years seem to be at greatest risk of stroke. Phlebotomy is generally
not recommended if symptoms of hyper-viscosity are absent and the hematocrit is
<65%.
Coagulation abnormalities are common in
patients with cyanotic heart disease. Platelet counts tend to be low-normal,
and many patients have subtle or overt defects in the coagulation cascade.
Phlebotomy may improve hemostasis in some patients. Hyperuricemia often occurs
because of increased urate reabsorption secondary to renal hypoperfusion. Gouty
arthritis is uncommon, but the hyperuricemia can result in progressive renal
impairment.
Preoperative Doppler echocardiography is
invaluable in helping to define the anatomy of the defect(s) and to confirm or
exclude the existence of other lesions or complications, their physiologi-cal
significance, and the effects of any therapeutic interventions.
Anesthetic Management
Th is population of patients includes four
groups: those who have undergone corrective cardiac sur-gery and require no
further operations, those who have had only palliative surgery, those who have
not yet undergone any cardiac surgery, and those whose conditions are
inoperable and may be awaiting car-diac transplantation. Although the
management of the first group of patients may be the same as that of normal
patients (except for consideration of prophy-lactic antibiotic therapy), the
care of others requires familiarity with the complex pathophysiology of these
defects. Even patients who have had corrective surgery may be prone to the
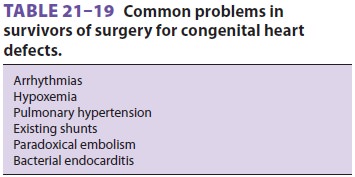
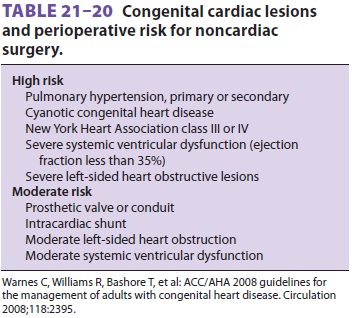
development of periop-erative problems (Tables 21–19 and 21–20). Some surgical procedures
eliminate the risk of endocar-ditis, whereas others increase the risk through
the use of prosthetic valves or conduits or the creation of new shunts.
For the purpose of anesthetic
management, con-genital heart defects may be divided into obstructive lesions,
predominantly left-to-right shunts, or pre-dominantly right-to-left shunts. In
reality, shunts can also be bidirectional and may reverse under cer-tain
conditions.
Related Topics