Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Cardiovascular Disease
Aortic Stenosis
AORTIC STENOSIS
Preoperative Considerations
Valvular aortic stenosis is the most
common cause of obstruction to left ventricular outflow. Left ven-tricular
outflow obstruction is less commonly due to hypertrophic cardiomyopathy,
discrete congenital subvalvular stenosis, or, rarely, supravalvular ste-nosis.
Valvular aortic stenosis is nearly always con-genital, rheumatic, or
degenerative. Abnormalities in the number of cusps (most commonly a bicuspid
valve) or their architecture produce turbulence that traumatizes the valve and
eventually leads to steno-sis. Rheumatic aortic stenosis is rarely isolated; it
is more commonly associated with aortic regurgita-tion or mitral valve disease.
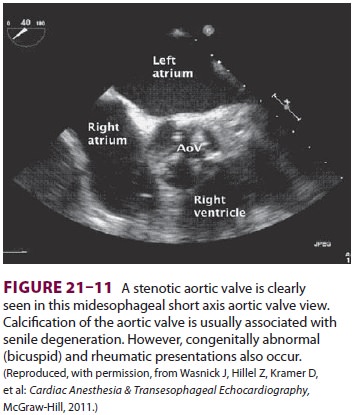
In the most common degenerative form, calcific aortic stenosis, wear and tear
results in the buildup of calcium deposits on normal cusps, preventing them
from opening com-pletely (Figure 21–11).
Pathophysiology
Left ventricular outflow obstruction caused
by val-vular aortic stenosis is almost always gradual, allow-ing the ventricle,
at least initially, to compensate and maintain SV. Concentric left ventricular
hypertrophy enables the ventricle to maintain SV by generating the needed
transvalvular pressure gradient and to reduce ventricular wall stress.
Critical aortic stenosis is said to
exist when the aortic valve orifice is reduced to 0.5–0.7 cm2 (normal is 2.5–3.5 cm2).
With this degree of stenosis, patients generally have a transvalvular gradient
of approxi-mately 50 mm Hg at rest (with a normal cardiac output) and are
unable to increase cardiac output in response to exertion. Moreover, further
increases in the transvalvular gradient do not significantly increase SV. With
long-standing aortic stenosis, myocardial contractility progressively
deteriorates and compromises left ventricular function.
Classically, patients with advanced
aortic ste-nosis have the triad of dyspnea on exertion, angina, and orthostatic
or exertional syncope. A promi-nent feature of aortic stenosis is a decrease in
left ventricular compliance as a result of hypertrophy. Diastolic dysfunction
is the result of an increase in ventricular muscle mass, fibrosis, or
myocardial ischemia. In contrast to left ventricular end-diastolic volume,
which remains normal until very late in the disease, left ventricular
end-diastolic pressure is elevated early in the disease. The decreased
dia-stolic pressure gradient between the left atrium and left ventricle impairs
ventricular filling, which becomes quite dependent on a normal atrial
con-traction. Loss of atrial systole can precipitate con-gestive heart failure
or hypotension in patients with aortic stenosis. Cardiac output may be normal
in symptomatic patients at rest, but characteristically, it does not
appropriately increase with exertion. Patients may experience angina even in
the absence of CAD. Myocardial oxygen demand increases because of ventricular
hypertrophy, whereas myo-cardial oxygen supply decreases as a result of the
marked compression of intramyocardial coronary vessels caused by high
intracavitary systolic pres-sures (up to 300 mm Hg). Exertional syncope or
near-syncope is thought to be due to an inability to tolerate the
vasodilatation in muscle tissue during exertion. Arrhythmias leading to severe
hypoper-fusion may also account for syncope and sudden death in some patients.
Related Topics