Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Cardiovascular Disease
Arrhythmias, Pacemakers, and Internal Cardioverter-Defibrillator Management
Arrhythmias,
Pacemakers, and Internal Cardioverter-Defibrillator Management
Electrolyte disorders, heart structure defects, inflam-mation, myocardial ischemia, cardiomyopathies, and conduction abnormalities can all contribute to the development of perioperative arrhythmias and heart block. Consequently, the anesthesia staff must be prepared to manage both chronic and new-onset cardiac rhythm problems.Supraventricular tachycardias (SVTs) can have hemodynamic consequences secondary to loss of AV synchrony and decreased diastolic filling time.
Loss of the “P” wave on the ECG with a
fast ven-tricular response is consistent with SVTs. Most SVTs occur secondary
to a reentrant mechanism. Reentrant arrhythmias occur when conduction tis-sues
in the heart depolarize or repolarize at varying rates. In this manner, a
self-perpetuating loop of repolarization and depolarization can occur in the
conduction pathways and/or AV node. SVTs pro-ducing hemodynamic collapse are
treated perioper-atively with synchronized cardioversion. Adenosine can
likewise be given to slow AV node conduction and potentially disrupt the
reentrant loop. SVTs
in patients without accessory conduction
bundles (Wolff–Parkinson–White [WPW] syndrome) are treated with β-blockers and
calcium channel block-ers. In patients with known WPW, procainamide or
amiodarone can be used to treat SVTs. At times, SVTs manifest with a broad QRS
complex and seem to be similar to VTs. Such rhythms, when they present, should
be treated like VT, until proven otherwise.
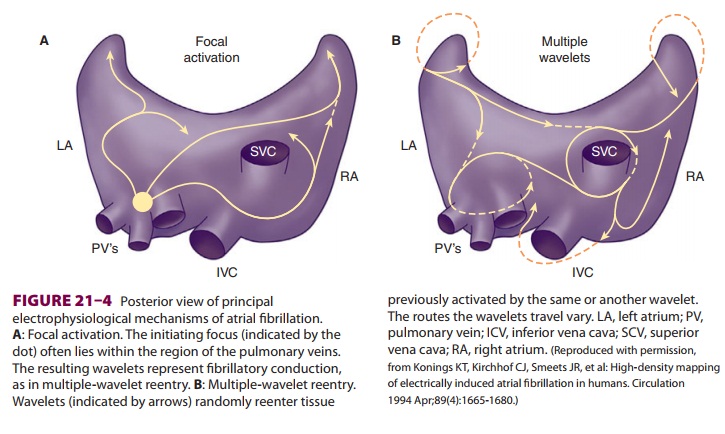
Atrial fibrillation (AF) can complicate
the peri-operative period (Figure 21–4) Up to 35% of car-diac surgery
patients develop postoperative AF. Moreover, many patients present with AF for
anes-thesia and noncardiac surgery. The ACC/AHA has issued voluminous
guidelines for the outpatient management of AF. The guidelines recommend use of
β-blockers or nondihydropyridine calcium antag-onists
for ventricular rate control in patients without accessory conduction pathways.
Amiodarone, pro-cainamide, disopyramide, and ibutilide are sug-gested for
ventricular rate control in patients with accessory pathways. The use of
digitalis and nondi-hyropryidine calcium channel blockers is contrain-dicated
in patients with accessory pathways.
The ACC/AHA guidelines also recommend
anti-thrombotic therapy in patients with long-standing AF. Consequently, many
patients with AF will pres-ent to the operating room on some form of
anti-thrombotic therapy—often the vitamin K antagonist warfarin. However, ACC/AHA
guidelines suggest that aspirin can be an alternative to vitamin K antag-onists
in low-risk patients or those with contraindi-cations to oral anticoagulation.
Likewise, in patients with AF without mechanical prosthetic heart valves, the
guidelines suggest that it is acceptable to discon-tinue anticoagulation for up
to 1 week in advance of surgical procedures, without instituting heparin
anticoagulation.When AF develops perioperatively, rate control with β-blockers can
often be instituted. Chemical cardioversion can be attempted with amiodarone or
procainamide. Of note, if the duration of AF is greater than 48 hours, or
unknown, ACC/AHA guidelines recommend anticoagulation for 3 weeks prior to and
4 weeks following either electrical or chemical cardioversion. Alternatively,
TEE can be performed to rule out the presence of left atrial or left atrial
appendage thrombus.
Should AF develop postoperatively,
ventricu-lar rate response can be controlled with AV nodal blocking agents,
unless contraindicated. Should AF result in hemodynamic instability,
synchronized cardioversion can be attempted. Patients at high risk of AF
following cardiac surgery can be treated with prophylactic amiodarone.
AF is most frequently associated with
loss of atrial muscle and the development of fibrosis. Fibrosis may contribute
to reentrant mechanisms of AF as depolarization/repolarization becomes
nonhomogeneous. AF may also develop from a focal source often located in the
pulmonary veins. In patients with an accessory bundle, AF can pro-duce rapid
ventricular responses and hemody-namic collapse. Drugs that slow conduction
across the AV node (eg, digitalis, verapamil, diltiazem) do not slow conduction
across the accessory pathway, potentially leading to hemodynamic collapse. The
ACC/AHA guidelines likewise recommend cau-tion in the use of β-blockers for AF
in patients with preexcitation syndromes.
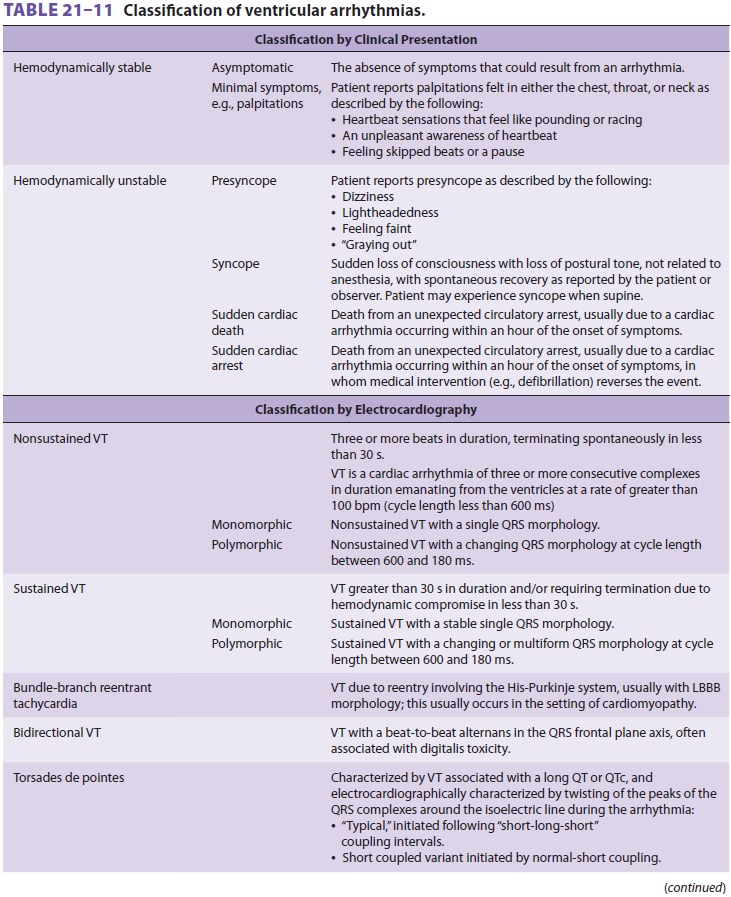
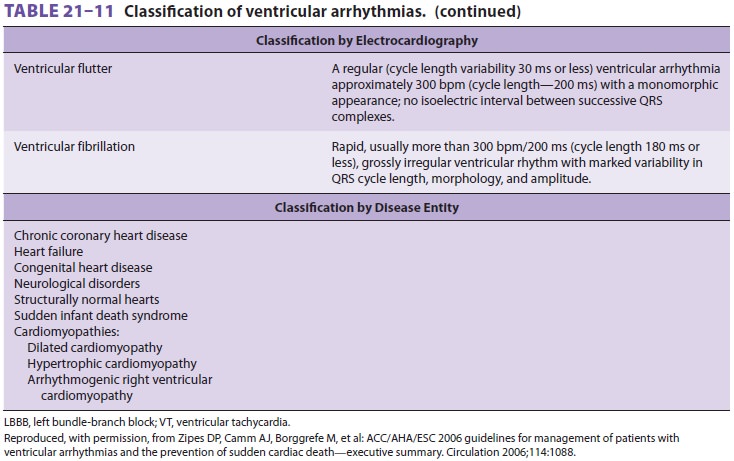
Ventricular arrhythmias have been the
sub-ject of much review by the AHA ( Table 21–11). Ventricular premature
contractions (VPCs) can appear perioperatively secondary to electrolyte
abnormalities (hypokalemia, hypomagnesium, hypocalcemia), acidosis, ischemia,
embolic phe-nomenon, mechanical irritation of the heart from central lines,
cardiac manipulation, and drug effects. Correction of the underlying source of
any arrhythmia should be addressed. Patients can likewise present with VPCs
secondary to vari-ous cardiomyopathies (dilated, hypertrophic, and
arrhythmogenic right ventricular).
The incidence of sudden cardiac death
(SCD) is estimated at 1-2/1000 per year. Consequently, some patients will
experience an unexpected death in the perioperative period. All anesthesia
providers must be prepared to resuscitate and manage patients with ventricular
arrhythmias, including VT (non-sustained and sustained) and ventricular
fibrillation.
Nonsustained ventricular tachycardia is
a short run of ventricular ectopy that lasts <30 sec and spon-taneously terminates,
whereas sustained VT persists longer than 30 seconds. VT is either monomorphic
or polymorphic, depending on the QRS complex. If the QRS complex morphology
changes, it is desig-nated as polymorphic VT. Torsades de pointes is a form of
VT associated with a prolonged QT inter-val, producing a sine wave-like VT
pattern on the ECG. Ventricular fibrillation requires immediate resuscitative
efforts and defibrillation.
Patients presenting with ventricular
ectopy and nonsustained runs of VT should undergo investiga-tion prior to
surgery. Supraventricular and ventricu-lar arrhythmias constitute active
cardiac conditions that warrant evaluation and treatment prior to elec-tive,
noncardiac surgery. Exercise testing, echo-cardiography, and nuclear perfusion
studies are all recommended by the ACC/AHA in patients with ventricular
arrhythmias as part of their workup and management. Electrophysiologic studies
are undertaken to determine the possibility for catheter-mediated ablation of
ventricular tachycardias.
Should VT present perioperatively,
cardiover-sion is recommended at any point where hemody-namic compromise
occurs. Otherwise, treatment with amiodarone or procainamide can be attempted.
At all times, therapy should also be directed at iden-tifying any causative
sources of the arrhythmia. β-Blockers are useful in the treatment of
VT, espe-cially if ischemia is a suspected causative factor in the development
of rhythm. The use of β-blockers following myocardial
infarction has reduced the incidence of post-MI ventricular fibrillation.
Torsades de pointes is associated with
condi-tions that lengthen the QT interval. If the arrhyth-mia develops in
association with pauses, pacing can be effective. Likewise, some patients may
benefit from isoproterenol infusions, if they develop pause-dependent torsades
de pointes. Magnesium sulfate may be useful in patients with long QT syndrome
and episodes of torsades.
The development of perioperative
ventricular fibrillation (VF) requires defibrillation and the use of
resuscitation algorithms. Amiodarone can be used to stabilize the rhythm following
successful defibrillation.Following VF, patients can present to sur-gery for
both ICD placement and other surgical procedures. ICDs are recommended in
patients with a history of survived sudden cardiac death (SCD), decreased
ventricular function following
MI, and left ventricular ejection
fractions <35%. Additionally, ICDs are used to
treat potential sud-den cardiac death in patients with dilated, hypertro-phic,
arrhythmogenic right ventricular, and genetic cardiomyopathies.
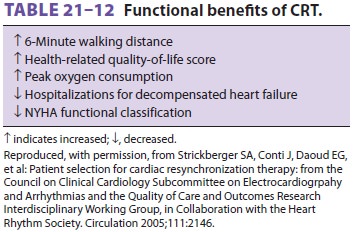
ICDs usually have a biventricular pacing
func-tion that improves the effectiveness of left ventricu-lar contraction.
Patients with heart failure frequently have a widened QRS complex >120 msec. In such patients, ventricular systole is
less efficient, as the lateral and septal left ventricular walls do not
effec-tively contract because of the conduction delay. Cardiac resynchronizaton
therapy (CRT) has been shown to improve functional status in patients with
heart failure (Table
21–12).
Anesthetic management for the placement of ICDs and other electrophysiologic procedures (eg, catheter ablation) depends on the patient’s under-lying conditions. Many patients present with sys-tolic and diastolic heart failure, and, as such, are dependent on sympathetic tone to maintain blood pressure. Many patients tolerate ICD placement using deep sedation rather than general anesthesia. However, catheter-based electrophysiologic stud-ies can be quite time consuming, and patients can develop atelectasis and airway obstruction. Should the patient’s blood pressure suddenly decline during electrophysiololgic studies, development of peri-cardial tamponade should be ruled out. Emergent drainage of tamponade may be necessary.
Many patients present to surgery with
ICDs in place. Published guidelines of the American Society of
Anesthesiologists can provide assistance in the management of such patients.
Management is a three-step process, as
follows:
·
Preoperative. Identify the type of device anddetermine if it is
used for antibradycardia functions. Consult with the patient’s cardiologist
preoperatively as to the device’s function and use history.
·
Intraoperative. Determine whatelectromagnetic
interference is likely to present intraoperatively and advise the use of
bipolar electrocautery where possible. Assure the availability of temporary
pacing and defibrillation equipment and apply pads as necessary. Patients who
are pacer dependent can be programmed to an asynchronous mode to mitigate
electrical interference. Magnet application to ICDs may disable the
antitachycardia function, but not convert to an asynchronous pacemaker.
Consultation with the patient’s cardiologist and interrogation of the device is
advised.
·
Postoperative. The device must be interrogatedto
ensure that therapeutic functions have been restored. Patients should be
continuously monitored until the antitachycardia functions of the device are restored
and its function has been confirmed.
ICDs are particularly problematic
intraop-eratively when electrocautery is used because the device may (1)
interpret cautery as ventricular fibril-lation; (2) inhibit pacemaker function
due to cautery artifact; (3) increase the pacing rate due to activation of a
rate-responsive sensor; or (4) temporarily or permanently reset to a backup or
reset mode. Use of bipolar cautery, placement of the grounding pad far from the
ICD device, and limiting use of the cautery to only short bursts help to reduce
the likelihood of problems, but will not eliminate them.
ICD devices should have the
defibrillator func-tion programmed off immediately before surgery and
reprogrammed back on immediately afterward. External defibrillation pads should
be applied and attached to a defibrillator machine intraoperatively.
Careful monitoring of the arterial pulse
with pulse oximetry or an arterial waveform is necessary to ensure that the
pacemaker is not inhibited and that there is arterial perfusion during episodes
of ECG artifact from surgical cautery. The manufacturer should be contacted to
determine the best method for managing the device (eg, reprogramming or
applying a magnet) prior to surgery. A large num-ber of ICD models are in use; however,
most sus-pend their antitachycardia function in response to a magnet.
Related Topics