Chapter: Medical Surgical Nursing: Management of Patients With Urinary Disorders
Chronic Renal Failure (End-Stage Renal Disease)
CHRONIC
RENAL FAILURE (END-STAGE RENAL DISEASE)
Chronic
renal failure, or ESRD, is a progressive, irreversible de-terioration in renal
function in which the body’s ability to main-tain metabolic and fluid and
electrolyte balance fails, resulting in uremia or azotemia (retention of urea
and other nitrogenous wastes in the blood).
The
incidence of ESRD has increased by almost 8% per year for the past 5 years,
with more than 300,000 patients being treated in the United States (USRDS,
2001).
ESRD
may be caused by systemic diseases, such as diabetes mellitus (leading cause);
hypertension; chronic glomerulonephri-tis; pyelonephritis; obstruction of the
urinary tract; hereditary le-sions, as in polycystic kidney disease; vascular
disorders; infections; medications; or toxic agents.
Autosomal
dominant polycystic kidney disease accounts for 8% to 10% of cases of ESRD in
the United States and Europe (Perrone, Ruthazer & Terrin, 2001). Comorbid conditions
that develop during chronic renal insufficiency contribute to the high
morbidity and mortality among patients with ESRD (Kausz et al., 2001).
Environmental
and occupational agents that have been impli-cated in chronic renal failure
include lead, cadmium, mercury, and chromium. Dialysis or kidney
transplantation eventually be-comes necessary for patient survival. Dialysis is
an effective means of correcting metabolic toxicities at any age, although the
mor-tality rate in infants and young children is greater than adults in the
presence of other, nonrenal diseases and in the presence of anuria or oliguria
(Wood et al., 2001).
Pathophysiology
As
renal function declines, the end products of protein metabo-lism (which are
normally excreted in urine) accumulate in the blood. Uremia develops and
adversely affects every system in the body. The greater the buildup of waste
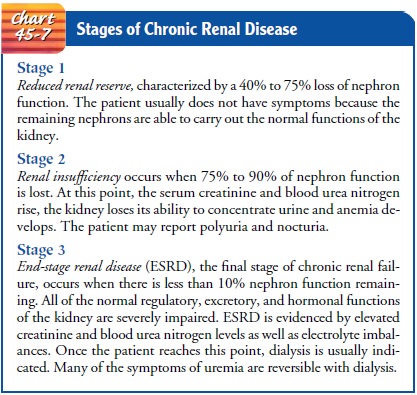
products, the more severe the symptoms. There are three well-recognized stages
of chronic renal disease: reduced renal reserve, renal insufficiency, and ESRD
(Chart 45-7).
The
rate of decline in renal function and progression of chronic renal failure is
related to the underlying disorder, the uri-nary excretion of protein, and the
presence of hypertension. The disease tends to progress more rapidly in
patients who excrete sig-nificant amounts of protein or have elevated blood
pressure than in those without these conditions.
Clinical Manifestations
Because
virtually every body system is affected by the uremia of chronic renal failure,
patients exhibit a number of signs and symptoms. The severity of these signs
and symptoms depends in part on the degree of renal impairment, other
underlying condi-tions, and the patient’s age.
CARDIOVASCULAR MANIFESTATIONS
Hypertension
(due to sodium and water retention or from activation of the
renin–angiotensin–aldosterone system), heart failure and pulmonary edema (due
to fluid overload), and peri-carditis (due to irritation of the pericardial
lining by uremic toxins) are among the cardiovascular problems manifested in
ESRD. Strict fluid volume control has been found to normalize hyper-tension in
patients receiving peritoneal dialysis (Gunal, Duman, Ozkahya et al., 2001).
Cardiovascular
disease is the predominant cause of death in patients with ESRD. In chronic
hemodialysis patients, approxi-mately 45% of overall mortality is attributable
to cardiac disease, and about 20% of these cardiac deaths are due to acute
myocar-dial infarction (USRDS, 2001).
DERMATOLOGIC SYMPTOMS
Severe itching (pruritus) is common. Uremic frost, the deposit of urea crystals on the skin, is uncommon today because of early and aggressive treatment of ESRD with dialysis.
OTHER SYSTEMIC MANIFESTATIONS
GI
signs and symptoms are common and include anorexia, nau-sea, vomiting, and
hiccups. Neurologic changes, including al-tered levels of consciousness,
inability to concentrate, muscle twitching, and seizures, have been observed.
The precise mecha-nisms for many of these diverse signs and symptoms have not
been identified. It is generally thought, however, that the accumu-lation of
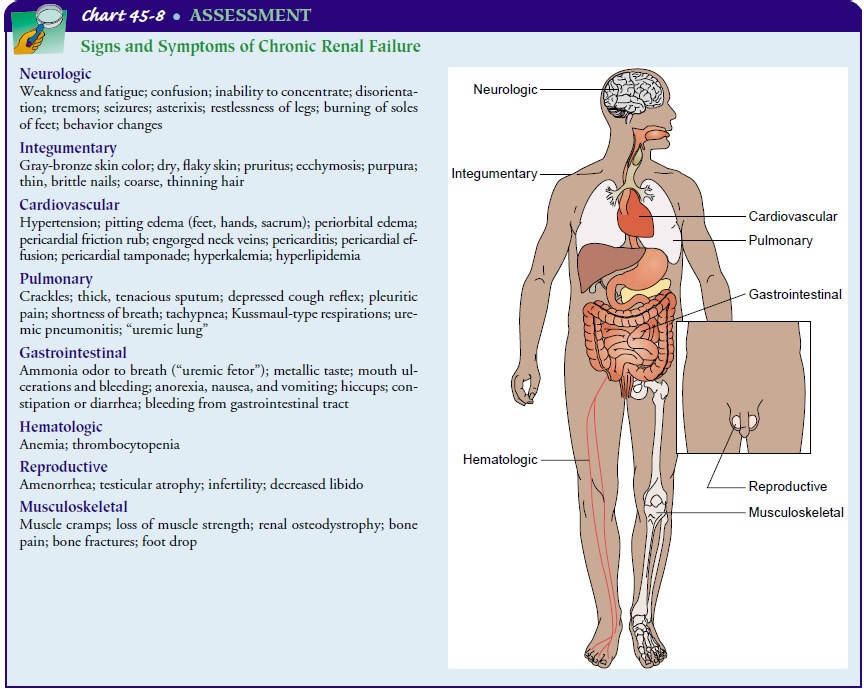
uremic waste products is the probable cause. Chart 45-8 summarizes the signs
and symptoms often seen in chronic renal failure.
Assessment and Diagnostic Findings
GLOMERULAR FILTRATION RATE
Decreased
GFR can be detected by obtaining a 24-hour urinaly-sis for creatinine
clearance. As glomerular filtration decreases (due to nonfunctioning
glomeruli), the creatinine clearance value de-creases, whereas the serum
creatinine and BUN levels increase. Serum creatinine is the more sensitive
indicator of renal function because of its constant production in the body. The
BUN is af-fected not only by renal disease but also by protein intake in the
diet, catabolism (tissue and RBC breakdown), parenteral nutri-tion, and
medications such as corticosteroids.
SODIUM AND WATER RETENTION
The
kidney cannot concentrate or dilute the urine normally in ESRD. Appropriate
responses by the kidney to changes in the daily intake of water and
electrolytes, therefore, do not occur. Some pa-tients retain sodium and water,
increasing the risk for edema, heart failure, and hypertension. Hypertension
may also result from ac-tivation of the renin–angiotensin–aldosterone axis and
the con-comitant increased aldosterone secretion. Other patients have a
tendency to lose salt and run the risk of developing hypotension and
hypovolemia. Episodes of vomiting and diarrhea may produce sodium and water
depletion, which worsens the uremic state.
ACIDOSIS
With advanced renal disease, metabolic
acidosis occurs because the kidney cannot excrete increased loads of acid.
Decreased acid secretion primarily results from inability of the kidney tubules
to excrete ammonia (NH3−) and to reabsorb sodium bicarbonate (HCO3−). There is also decreased excretion of phosphates
and other organic acids.
ANEMIA
Anemia develops as a result of inadequate erythropoietin pro-duction, the shortened life span of RBCs, nutritional deficiencies, and the patient’s tendency to bleed, particularly from the GI tract. Erythropoietin, a substance normally produced by the kid-ney, stimulates bone marrow to produce RBCs. In renal failure, erythropoietin production decreases and profound anemia re-sults, producing fatigue, angina, and shortness of breath.
CALCIUM AND PHOSPHORUS IMBALANCE
Another
major abnormality seen in chronic renal failure is a dis-order in calcium and
phosphorus metabolism. Serum calcium and phosphate levels have a reciprocal
relationship in the body: as one rises, the other decreases. With decreased
filtration through the glomerulus of the kidney, there is an increase in the
serum phosphate level and a reciprocal or corresponding decrease in the serum calcium
level. The decreased serum calcium level causes in-creased secretion of
parathormone from the parathyroid glands. In renal failure, however, the body
does not respond normally to the increased secretion of parathormone; as a
result, calcium leaves the bone, often producing bone changes and bone disease.
In addition, the active metabolite of vitamin D
(1,25-dihydroxy-cholecalciferol) normally manufactured by the kidney decreases
as renal failure progresses. Uremic bone disease, often called renal osteodystrophy,
develops from the complex changes in calcium, phosphate, and parathormone
balance (Barnas, Schmidt, Seidl et al., 2001).
Complications
Potential
complications of chronic renal failure that concern the nurse and that
necessitate a collaborative approach to care include the following:
· Hyperkalemia due to
decreased excretion, metabolic acido-sis, catabolism, and excessive intake
(diet, medications, fluids)
· Pericarditis,
pericardial effusion, and pericardial tamponade due to retention of uremic waste
products and inadequate dialysis
· Hypertension due to
sodium and water retention and mal-function of the
renin–angiotensin–aldosterone system
· Anemia due to decreased
erythropoietin production, de-creased RBC life span, bleeding in the GI tract
from irritat-ing toxins, and blood loss during hemodialysis
· Bone disease and
metastatic calcifications due to retention of phosphorus, low serum calcium
levels, abnormal vitamin D metabolism, and elevated aluminum levels
Medical Management
The
goal of management is to maintain kidney function and homeostasis for as long
as possible. All factors that contribute to ESRD and all factors that are
reversible (eg, obstruction) are iden-tified and treated. Management is
accomplished primarily with medications and diet therapy, although dialysis may
also be needed to decrease the level of uremic waste products in the blood
(Fink et al., 2001).
PHARMACOLOGIC THERAPY
Complications
can be prevented or delayed by administering pre-scribed antihypertensives,
erythropoietin (Epogen), iron supple-ments, phosphate-binding agents, and
calcium supplements.
Antacids.
Hyperphosphatemia and
hypocalcemia are treatedwith aluminum-based antacids that bind dietary
phosphorus in the GI tract. However, concerns about the potential long-term toxicity of aluminum and
the association of high aluminum levels with neurologic symptoms and
osteomalacia have led some physi-cians to prescribe calcium carbonate in place
of high doses of aluminum-based antacids. This medication also binds dietary
phosphorus in the intestinal tract and permits the use of smaller doses of
antacids. Both calcium carbonate and phosphorus-binding antacids must be
administered with food to be effective. Magnesium-based antacids must be
avoided to prevent magnesium toxicity.
Antihypertensive and Cardiovascular Agents.
Hypertension ismanaged by intravascular volume control and a variety of
anti-hypertensive agents. Heart failure and pulmonary edema may also require
treatment with fluid restriction, low-sodium diets, diuretic agents, inotropic
agents such as digitalis or dobutamine, and dialysis. The metabolic acidosis of
chronic renal failure usu-ally produces no symptoms and requires no treatment;
however, sodium bicarbonate supplements or dialysis may be needed to correct the
acidosis if it causes symptoms (Tonelli et al., 2001).
Antiseizure Agents.
Neurologic
abnormalities may occur, so thepatient must be observed for early evidence of
slight twitching, headache, delirium, or seizure activity. If seizures occur,
the onset of the seizure is recorded along with the type, duration, and
gen-eral effect on the patient. The physician is notified immediately.
Intravenous diazepam (Valium) or phenytoin (Dilantin) is usu-ally administered
to control seizures. The side rails of the bed should be padded to protect the
patient.
Erythropoietin.
Anemia
associated with chronic renal failure istreated with recombinant human
erythropoietin (Epogen). Ane-mic patients (hematocrit less than 30%) present
with nonspecific symptoms, such as malaise, general fatigability, and decreased
ac-tivity tolerance. Epogen therapy is initiated to achieve a hemat-ocrit of
33% to 38%, which generally alleviates the symptoms of anemia. Epogen is
administered either intravenously or subcuta-neously three times a week. It may
take 2 to 6 weeks for the hemat-ocrit to rise; therefore, Epogen is not
indicated for patients who need immediate correction of severe anemia. Adverse
effects seen with Epogen therapy include hypertension (especially during early
stages of treatment), increased clotting of vascular access sites, seizures,
and depletion of body iron stores (Fink et al., 2001).
The
patient receiving Epogen may experience influenza-like symptoms with initiation
of therapy; these tend to subside with repeated doses. Management involves
adjustment of heparin to prevent clotting of the dialysis lines during
hemodialysis treat-ments, frequent monitoring of hematocrit, and periodic
assess-ment of serum iron and transferrin levels. Because adequate stores of
iron are necessary for an adequate response to erythropoietin, supplementary
iron may be prescribed. In addition, the patient’s blood pressure and serum
potassium level are monitored to detect hypertension and rising serum potassium
levels, which may occur with therapy and the increasing RBC mass. The
occurrence of hy-pertension requires initiation or adjustment of the patient’s
anti-hypertensive therapy. Hypertension that cannot be controlled is a
contraindication to recombinant erythropoietin therapy.
Patients
who have received Epogen have reported decreased levels of fatigue, an
increased feeling of well-being, better tolerance of dialysis, higher energy
levels, and improved exercise tolerance. Additionally, this therapy has
decreased the need for transfusion and its associated risks, including
bloodborne infectious disease, antibody formation, and iron overload (Fink et
al., 2001).
NUTRITIONAL THERAPY
Dietary
intervention is necessary with deterioration of renal func-tion and includes
careful regulation of protein intake, fluid intake to balance fluid losses,
sodium intake to balance sodium losses, and some restriction of potassium. At
the same time, adequate caloric intake and vitamin supplementation must be
ensured. Pro-tein is restricted because urea, uric acid, and organic acids—the
breakdown products of dietary and tissue proteins—accumulate rapidly in the
blood when there is impaired renal clearance. The allowed protein must be of
high biologic value (dairy prod-ucts, eggs, meats). High-biologic-value proteins
are those that are complete proteins and supply the essential amino acids
necessary for growth and cell repair.
Usually,
the fluid allowance is 500 to 600 mL more than the previous day’s 24-hour urine
output. Calories are supplied by car-bohydrates and fat to prevent wasting.
Vitamin supplementation is necessary because a protein-restricted diet does not
provide the necessary complement of vitamins. Additionally, the patient on
dialysis may lose water-soluble vitamins from the blood during the dialysis treatment.
OTHER THERAPY: DIALYSIS
Hyperkalemia
is usually prevented by ensuring adequate dialysis treatments with potassium
removal and careful monitoring of all medications, both oral and intravenous,
for their potassium con-tent. The patient is placed on a potassium-restricted
diet. Occa-sionally, Kayexalate, a cation-exchange resin, administered orally,
may be needed. The patient with increasing symptoms of chronic renal failure is
referred to a dialysis and transplantation center early in the course of
progressive renal disease. Dialysis is usually initiated when the patient
cannot maintain a reasonable lifestyle with conservative treatment.
Nursing Management
The
patient with chronic renal failure requires astute nursing care to avoid the
complications of reduced renal function and the stresses and anxieties of
dealing with a life-threatening illness. Ex-amples of potential nursing
diagnoses for these patients include the following:
· Excess fluid volume
related to decreased urine output, dietary excesses, and retention of sodium
and water
· Imbalanced nutrition:
less than body requirements related to anorexia, nausea and vomiting, dietary
restrictions, and altered oral mucous membranes
· Deficient knowledge
regarding condition and treatment regimen
· Activity intolerance
related to fatigue, anemia, retention of waste products, and dialysis procedure
· Low self-esteem related
to dependency, role changes, changes in body image, and sexual dysfunction
Nursing
care is directed toward assessing fluid status and iden-tifying potential
sources of imbalance, implementing a dietary program to ensure proper
nutritional intake within the limits of the treatment regimen, and promoting
positive feelings by en-couraging increased self-care and greater independence.
It is ex-tremely important to provide explanations and information to the
patient and family concerning ESRD, treatment options, and potential
complications. A great deal of emotional support is needed by the patient and
family because of the numerous changes experienced. Specific interventions,
along with rationale and evaluation criteria, are presented in more detail in
the Plan of Nursing Care.
PROMOTING HOME AND COMMUNITY-BASED CARE
Teaching Patients Self-Care.
The nurse
plays an extremely im-portant role in teaching the patient with ESRD. Because
of the extensive teaching needed, the home care nurse, dialysis nurse, and
nurse in the outpatient setting all provide ongoing education and reinforcement
while monitoring the patient’s progress and compliance with the treatment
regimen.
A
nutritional referral and explanations of nutritional needs are helpful because
of the numerous dietary changes required. The patient is taught how to check
the vascular access device for pa-tency and how to take precautions, such as
avoiding venipunc-tures and blood pressure measurements on the arm with the
access device.
Additionally,
the patient and family require considerable as-sistance and support in dealing
with the need for dialysis and its long-term implications. For instance, they
need to know what problems to report to the health care provider, including the
following:
· Worsening signs and
symptoms of renal failure (nausea, vomiting, change in usual urine output [if
any], ammonia odor on breath)
· Signs and symptoms of hyperkalemia
(muscle weakness, diarrhea, abdominal cramps)
· Signs and symptoms of
access problems (clotted fistula or graft, infection)
These
signs and symptoms of decreasing renal function, in ad-dition to increasing BUN
and serum creatinine levels, may indi-cate a need to alter the dialysis
prescription. The dialysis nurses also provide ongoing education and support at
each treatment visit.
Continuing Care.
The
importance of follow-up examinationsand treatment is stressed to the patient
and family because of changing physical status, renal function, and dialysis
require-ments. Referral for home care provides the home care nurse with the
opportunity to assess the patient’s environment, emotional status, and the
coping strategies used by the patient and family to deal with the changes in
family roles often associated with chronic illness.
The
home care nurse also assesses the patient for further dete-rioration of renal
function and signs and symptoms of complica-tions resulting from the primary
renal disorder, the resulting renal failure, and effects of treatment
strategies (eg, dialysis, medica-tions, dietary restrictions). Many patients
need ongoing education and reinforcement on the multiple dietary restrictions
required, including fluid, sodium, potassium, and protein restriction.
Reminders about the need for health promotion activities and health screening
are an important part of nursing care for the patient with renal failure.
Gerontologic Considerations
Changes in kidney function with normal aging increase the sus-ceptibility of elderly patients to kidney dysfunction and renal fail-ure. Because alterations in renal blood flow, glomerular filtration, and renal clearance increase the risk for medication-associated changes in renal function, precautions are indicated with all medications.
This is because of the frequent use of multiple-prescription and
over-the-counter medications by elderly patients. The incidence of systemic
diseases, such as atherosclerosis, hyper-tension, heart failure, diabetes, and
cancer, increases with advanc-ing age, predisposing older adults to renal
disease associated with these disorders. Therefore, nurses in all settings need
to be alert for signs and symptoms of renal dysfunction in elderly patients.
With
age, the kidney is less able to respond to acute fluid and electrolyte changes.
Therefore, acute problems need to be pre-vented if possible or recognized and
treated quickly to avoid kid-ney damage. When the elderly patient must undergo
extensive diagnostic tests, or when new medications (eg, diuretic agents) are
added, precautions must be taken to prevent dehydration, which can compromise
marginal renal function and lead to ARF.
The
elderly patient may develop atypical and nonspecific signs and symptoms of
disturbed renal function and fluid and elec-trolyte imbalances. Recognition of
these problems is further ham-pered by their association with preexisting
disorders and the misconception that they are normal changes of aging.
ACUTE RENAL FAILURE IN OLDER ADULTS
The
incidence of ARF is increasing in older, hospitalized patients. About half of
patients who develop ARF during hospitalization for a medical or surgical
problem are older than 60 years of age. Evidence also demonstrates that ARF is
often seen in the com-munity setting. Nurses in the ambulatory setting need to
be cog-nizant of the risk for ARF in their elderly patients, especially those
undergoing diagnostic testing or procedures that can result in de-hydration.
The mortality rate is slightly higher for ARF in elderly patients than for
their younger counterparts.
The
etiology of ARF in older adults includes prerenal causes, such as dehydration,
and intrarenal causes, such as nephrotoxic agents (medications, contrast
agents). Diabetes mellitus increases the risk for contrast agent-induced renal
failure because of pre-existing renal insufficiency and the imposed fluid
restriction needed for many tests. Suppression of thirst, enforced bed rest,
lack of drinking water, and confusion all contribute to the older patient’s
failure to consume adequate fluids and may lead to dehydration and compromise
of already decreased renal function.
CHRONIC RENAL FAILURE IN OLDER ADULTS
Historically,
the age of patients developing ESRD steadily rose each year, but it appears to
have stabilized since 1993 at a mean age of 60 years. In the past, rapidly
progressive glomerulonephri-tis, membranous glomerulonephritis, and
nephrosclerosis were the most common causes of chronic renal failure in the
elderly. Today, however, diabetes mellitus and hypertension are the lead-ing
causes of chronic renal failure in the elderly (Bakris et al., 2000). Other
common causes of chronic renal failure in the el-derly population are
interstitial nephritis and urinary tract ob-struction. The signs and symptoms
of renal disease in the elderly are commonly nonspecific. The occurrence of
symptoms of other disorders (heart failure, dementia) can mask the symptoms of
renal disease and delay or prevent diagnosis and treatment. The patient often
develops signs and symptoms of nephrotic syn-drome, such as edema and
proteinuria.
Hemodialysis
and peritoneal dialysis have been used effec-tively in treating elderly
patients (Carey et al., 2001). Although there is no specific age limitation for
renal transplantation, con-comitant disorders (ie, coronary artery disease,
peripheral vascu-lar disease) have made it a less common treatment for the elderly.
The
outcome, however, is comparable to that of younger pa-tients. Some elderly
patients elect not to participate in these treatment strategies. Conservative
management, including nutri-tional therapy, fluid control, and medications,
such as phosphate binders, may be considered in patients who are not suitable
for or elect not to participate in dialysis or transplantation.
Related Topics