Chapter: Medical Surgical Nursing: Management of Patients With Urinary Disorders
Acute Renal Failure
Renal Failure
Renal
failure results when the kidneys cannot remove the body’s metabolic wastes or
perform their regulatory functions. The sub-stances normally eliminated in the
urine accumulate in the body fluids as a result of impaired renal excretion,
leading to a disrup-tion in endocrine and metabolic functions as well as fluid,
elec-trolyte, and acid–base disturbances. Renal failure is a systemic disease
and is a final common pathway of many different kidney and urinary tract
diseases. Each year, the number of deaths from irreversible renal failure
increases (U.S. Renal Data System, 2001).
ACUTE
RENAL FAILURE
Pathophysiology
Acute
renal failure (ARF) is a sudden and almost complete loss of kidney function (decreased
GFR) over a period of hours to days. Although ARF is often thought of as a
problem seen only in hos-pitalized patients, it may occur in the outpatient
setting as well. ARF manifests with oliguria, anuria, or normal urine volume.
Oliguria (less than 400 mL/day of urine) is the most common clinical situation
seen in ARF; anuria (less than 50 mL/day of urine) and normal urine output are
not as common. Regardless of the volume of urine excreted, the patient with ARF
experiences rising serum creatinine and BUN levels and retention of other
metabolic waste products (azotemia) normally excreted by the kidneys.
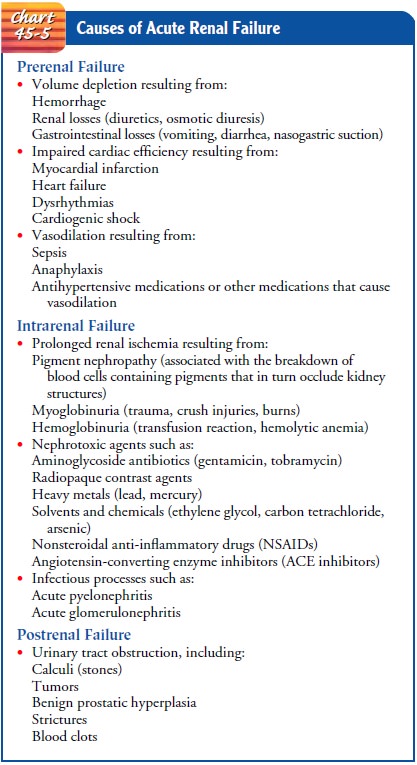
CATEGORIES OF ACUTE RENAL FAILURE
Three
major categories of conditions cause ARF: prerenal (hypo-perfusion of kidney),
intrarenal (actual damage to kidney tissue), and postrenal (obstruction to
urine flow).
· Prerenal conditions
occur as a result of impaired blood flow that leads to hypoperfusion of the
kidney and a drop in the GFR. Common clinical situations are volume-depletion
states (hemorrhage or GI losses), impaired cardiac perfor-mance (myocardial
infarction, heart failure, or cardiogenic shock), and vasodilation (sepsis or
anaphylaxis).
·
Intrarenal causes of ARF are the result of actual
parenchymal damage to the glomeruli or kidney tubules. Conditions such as
burns, crush injuries, and infections, as well as nephrotoxic agents, may lead
to acute tubular necrosis and
cessation of renal function. With burns and crush injuries, myoglobin (a
protein released from muscle when injury occurs) and he-moglobin are liberated,
causing renal toxicity, ischemia, or both. Severe transfusion reactions may
also cause intrarenal failure; hemoglobin is released through hemolysis,
filters through the glomeruli, and becomes concentrated in the kidney tubules
to such a degree that precipitation of hemo-globin occurs. Medications may also
predispose a patient to intrarenal damage, especially nonsteroidal
anti-inflammatory drugs (NSAIDs) and ACE inhibitors. These medications
interfere with the normal autoregulatory mechanisms of the kidney and may cause
hypoperfusion and eventual is-chemia. Other potential causes of intrarenal or
intrinsic ARF include rhabdomyolysis, which results in accumula-tion of
myoglobin in the glomeruli secondary to damage to skeletal muscle, and
nephrotoxicity secondary to herbal remedies (Myhre, 2000).
· Postrenal causes of ARF
are usually the result of an obstruc-tion somewhere distal to the kidney.
Pressure rises in the kid-ney tubules; eventually, the GFR decreases.
Common causes of ARF are summarized in Chart 45-5. Although the exact pathogenesis of ARF and oliguria is not always known, many times there is a specific underlying problem. Some of the factors may be reversible if identified and treated promptly, before kidney function is impaired. This is true of the following conditions that reduce blood flow to the kidney and impair kidney function: (1) hypovolemia; (2) hypotension; (3) re-duced cardiac output and heart failure; (4) obstruction of the kidney or lower urinary tract by tumor, blood clot, or kidney stone; and (5) bilateral obstruction of the renal arteries or veins. If these conditions are treated and corrected before the kidneys are permanently damaged, the increased BUN and creatinine lev-els, oliguria, and other signs associated with ARF may be reversed.
Although
not a common cause of ARF, some types of renal stones may increase the risk for
ARF more than others. Heredi-tary stone diseases (cystinuria, primary
hyperoxaluria, Dent’s dis-ease), primary struvite stones, and infection-related
urolithiasis associated with anatomic and functional urinary tract anomalies
and spinal cord injury may cause recurrent bouts of obstruction as well as
crystal-specific effects on tubular epithelial cells and in-terstitial renal
cells. This in turn may activate the fibrogenic cas-cade responsible for the
loss of renal parenchyma (Gambaro, Favaro & D’Angelo, 2001).
PHASES OF ACUTE RENAL FAILURE
There
are four clinical phases of ARF: initiation, oliguria, diure-sis, and recovery.
The initiation period begins with the initial in-sult and ends when oliguria
develops. The oliguria period is accompanied by a rise in the serum
concentration of substances usually excreted by the kidneys (urea, creatinine,
uric acid, or-ganic acids, and the intracellular cations [potassium and
magne-sium]). The minimum amount of urine needed to rid the body of normal
metabolic waste products is 400 mL. In this phase ure-mic symptoms first appear
and life-threatening conditions such as hyperkalemia develop.
Some
patients have decreased renal function with increasing nitrogen retention, yet
actually excrete normal amounts of urine (2 L/day or more). This is the
nonoliguric form of renal failure and occurs predominantly after nephrotoxic
antibiotic agents are administered to the patient; it may occur with burns,
traumatic injury, and the use of halogenated anesthetic agents.
In the
diuresis period, the third phase, the patient experiences gradually increasing
urine output, which signals that glomer-ular filtration has started to recover.
Laboratory values stop rising and eventually decrease. Although the volume of
urinary output may reach normal or elevated levels, renal function may still be
markedly abnormal. Because uremic symptoms may still be pres-ent, the need for
expert medical and nursing management con-tinues. The patient must be observed
closely for dehydration during this phase; if dehydration occurs, the uremic
symptoms are likely to increase.
The
recovery period signals the improvement of renal func-tion and may take 3 to 12
months. Laboratory values return to the patient’s normal level. Although a
permanent 1% to 3% re-duction in the GFR is common, it is not clinically
significant.
Clinical Manifestations
Almost
every system of the body is affected when there is failure of the normal renal
regulatory mechanisms. The patient may appear critically ill and lethargic,
with persistent nausea, vomit-ing, and diarrhea. The skin and mucous membranes
are dry from dehydration, and the breath may have the odor of urine (uremic
fetor). Central nervous system signs and symptoms in-clude drowsiness,
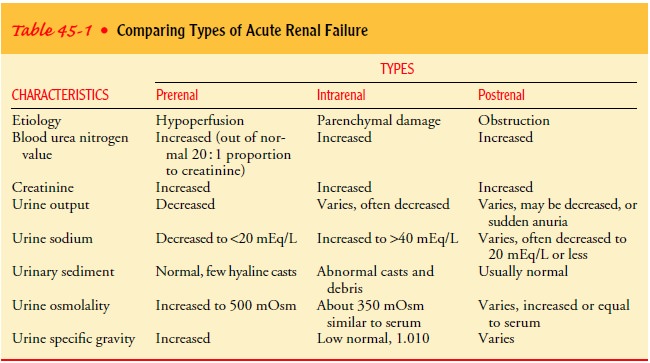
headache, muscle twitching, and seizures. Table 45-1 summarizes common clinical
findings for all three categories of ARF.
Assessment and Diagnostic Findings
CHANGES IN URINE
Urine output varies (scanty to normal volume), hematuria may be present, and the urine has a low specific gravity (1.010 or less, compared with a normal value of 1.015 to 1.025). Patients with prerenal
azotemia have a decreased amount of sodium in the urine (below 20 mEq/L) and
normal urinary sediment. Patients with intrarenal azotemia usually have urinary
sodium levels greater than 40 mEq/L with casts and other cellular debris.
Uri-nary casts are mucoproteins secreted by the renal tubules when-ever
inflammation is present.
CHANGE IN KIDNEY CONTOUR
Ultrasonography
is a critical component of the evaluation of both acute and chronic renal
failure. Although many sonographic find-ings are nonspecific, their diagnostic
utility is greatly enhanced by a familiarity with the clinical presentation and
a thorough under-standing of renal pathophysiology (O’Neill, 2000).
INCREASED BUN AND CREATININE LEVELS (AZOTEMIA)
The
BUN level rises steadily at a rate dependent on the degree of catabolism
(breakdown of protein), renal perfusion, and protein intake. Serum creatinine
rises in conjunction with glomerular damage. Serum creatinine levels are useful
in monitoring kidney function and disease progression.
HYPERKALEMIA
With a
decline in the GFR, the patient cannot excrete potassium normally. Patients
with oliguria and anuria are at greater risk
for hyperkalemia than those without oliguria. Protein catabolism re-sults in
the release of cellular potassium into the body fluids, caus-ing severe
hyperkalemia (high serum K+ levels). Hyperkalemia may lead to dysrhythmias and cardiac
arrest. Sources of potas-sium include normal tissue catabolism, dietary intake,
blood in the GI tract, or blood transfusion and other sources (intravenous
infusions, potassium penicillin, and extracellular shift in response to
metabolic acidosis).
METABOLIC ACIDOSIS
Patients
with acute oliguria cannot eliminate the daily metabolic load of acid-type
substances produced by the normal metabolic processes. In addition, normal
renal buffering mechanisms fail. This is reflected by a fall in the serum CO2-combining power and
blood pH. Thus, progressive metabolic acidosis accompanies renal failure.
CALCIUM AND PHOSPHORUS ABNORMALITIES
There
may be an increase in serum phosphate concentrations; serum calcium levels may
be low in response to decreased ab-sorption of calcium from the intestine and
as a compensatory mechanism for the elevated serum phosphate levels.
ANEMIA
Anemia
inevitably accompanies ARF due to reduced erythropoi-etin production, uremic GI
lesions, reduced RBC life span, and blood loss, usually from the GI tract. With
use of the parenteral form of erythropoietin (Epogen), anemia is not the major
prob-lem it once was.
Prevention
A
careful history is obtained to determine whether the patient has been taking
potentially nephrotoxic antibiotic agents or has been exposed to environmental
toxins. The kidneys are especially sus-ceptible to the adverse effects of
medications because the kidneys are repeatedly exposed to substances in the
blood. They receive a large blood flow (25% of the cardiac output at rest; the
entire blood volume circulates through the kidneys about 14 times a minute). In
addition, the kidney is the major excretory organ for many toxic substances,
and during the normal urine concentra-tion process, these substances increase
in concentration and can be toxic to the kidneys. Therefore, in patients taking
potentially nephrotoxic medications (aminoglycosides, gentamicin, tobramy-cin,
colistimethate, polymyxin B, amphotericin B, vancomycin, amikacin,
cyclosporine), renal function should be monitored closely. Serum BUN and
creatinine levels should be obtained at baseline by 24 hours after initiation
of these medications and at least twice a week while the patient is receiving
them.
Any
agent that reduces renal blood flow (eg, chronic analgesic use) may cause renal
insufficiency. Chronic analgesic use, partic-ularly with NSAIDs, may cause
interstitial nephritis and papil-lary necrosis. Patients with heart failure or
cirrhosis with ascites are at particular risk for NSAID-induced renal failure.
Increased age, preexisting renal disease, and the administration of several
nephrotoxic agents simultaneously increase the risk for kidney damage.
Management
of ARF is expensive and complex, and even when optimal, the mortality rate
remains high. Therefore, pre-vention of ARF is key (Chart 45-6).

Medical Management
The
kidney has a remarkable ability to recover from insult. Therefore, the
objectives of treatment of ARF are to restore nor-mal chemical balance and
prevent complications until repair of renal tissue and restoration of renal
function can take place. Any possible cause of damage is identified, treated,
and eliminated. Prerenal azotemia is treated by optimizing renal perfusion,
whereas postrenal failure is treated by relieving the obstruction. Treatment of
intrarenal azotemia is supportive, with removal of causative agents, aggressive
management of prerenal and postrenal failure, and avoidance of associated risk
factors. Shock and infection, if present, are treated promptly. Overall,
medical management in-cludes maintaining fluid balance, avoiding fluid
excesses, or pos-sibly performing dialysis.
Maintenance
of fluid balance is based on daily body weight, serial measurements of central
venous pressure, serum and urine concentrations, fluid losses, blood pressure,
and the clinical sta-tus of the patient. The parenteral and oral intake and the
output of urine, gastric drainage, stools, wound drainage, and perspira-tion
are calculated and are used as the basis for fluid replacement. The insensible
fluid lost through the skin and lungs and produced through the normal metabolic
processes is also considered in fluid management.
Fluid excesses can be detected by the clinical findings of dys-pnea, tachycardia, and distended neck veins. The lungs are auscultated
for moist crackles. Because pulmonary edema may be caused by excessive
administration of parenteral fluids, extreme caution must be used to prevent
fluid overload. The development of gen-eralized edema is assessed by examining
the presacral and pre-tibial areas several times daily. Mannitol, furosemide,
or ethacrynic acid may be prescribed to initiate a diuresis and prevent or
mini-mize subsequent renal failure.
Adequate
blood flow to the kidneys in patients with prerenal causes of ARF may be
restored by intravenous fluids or blood product transfusions. If ARF is caused
by hypovolemia secondary to hypoproteinemia, an infusion of albumin may be
prescribed. Dialysis may be initiated to prevent serious complications of ARF,
such as hyperkalemia, severe metabolic acidosis, pericardi-tis, and pulmonary
edema. Dialysis corrects many biochemical abnormalities; allows for
liberalization of fluid, protein, and sodium intake; diminishes bleeding
tendencies; and may help wound healing. Hemodialysis, peritoneal dialysis, or
any of the new continuous renal replacement therapies may be performed.
PHARMACOLOGIC THERAPY
Because
hyperkalemia is the most life-threatening of the fluid and electrolyte
disturbances, the patient is monitored for hyper-kalemia through serial serum
electrolyte levels (potassium value more than 5.5 mEq/L [5.5 mmol/L]),
electrocardiogram changes (tall, tented, or peaked T waves), and changes in
clinical status.
The elevated
potassium levels may be reduced by administering cation-exchange resins (sodium
polystyrene sulfonate [Kayexalate]) orally or by retention enema. Kayexalate
works by exchanging a sodium ion for a potassium ion in the intestinal tract.
Sorbitol is often administered in combination with Kayexalate to induce a
diarrhea-type effect (it induces water loss in the GI tract).
If a
retention enema is administered (the colon is the major site for potassium
exchange), a rectal catheter with a balloon may be used to facilitate retention
if necessary. The patient should re-tain the resin 30 to 45 minutes to promote
potassium removal. Afterward, a cleansing enema may be prescribed to remove the
Kayexalate resin as a precaution against fecal impaction.
Because
many medications are eliminated through the kidneys, medication dosages must be
reduced when a patient has ARF. Ex-amples of commonly used medications that
require adjustment are antibiotic agents (especially aminoglycosides), digoxin,
ACE inhibitors, and medications containing magnesium.
Many
medications have been used in patients with ARF in an attempt to improve
patient outcomes. Diuretic agents are often used to control fluid volume, but
they have not been shown to hasten the recovery from ARF.
Low-dose
dopamine (1 to 3 g/kg) is often used to dilate the renal arteries through
stimulation of dopaminergic receptors; however, research has not definitely
demonstrated that dopamine prevents ARF or improves outcome in patients with
established renal failure.
Atrial
natriuretic peptide (ANP), an endogenous hormone synthesized by the cardiac
atria, has been shown to improve renal function in multiple animal models of
ARF. It has also decreased the need for dialysis in patients with oliguric
acute tubular necro-sis in a multisite clinical trial of patients. Patients
with nonoliguric acute tubular necrosis did not benefit (Lewis, Salem, Chertow
et al., 2000). Further research on ANP use is underway.
In
patients with severe acidosis, the arterial blood gases or serum bicarbonate
levels (CO2-combining power) must
be mon-itored because the patient may require sodium bicarbonate therapy or
dialysis. If respiratory problems develop, appropriate ventilatory measures
must be instituted. The elevated serum phosphate level may be controlled with
phosphate-binding agents (aluminum hy-droxide). These agents help prevent a
continuing rise in serum phosphate levels by decreasing the absorption of
phosphate from the intestinal tract.
NUTRITIONAL THERAPY
ARF
causes severe nutritional imbalances (because nausea and vomiting contribute to
inadequate dietary intake), impaired glu-cose use and protein synthesis, and
increased tissue catabolism. The patient is weighed daily and can be expected
to lose 0.2 to 0.5 kg (0.5 to 1 lb) daily if the nitrogen balance is negative
(ie, the patient’s caloric intake falls below caloric requirements). If the
patient gains or does not lose weight or develops hypertension, fluid retention
should be suspected.
Dietary
proteins are limited to about 1 g/kg during the oliguric phase to minimize
protein breakdown and to prevent accumula-tion of toxic end products. Caloric
requirements are met with high-carbohydrate meals because carbohydrates have a
protein-sparing effect (ie, in a high-carbohydrate diet, protein is not used
for meeting energy requirements but is “spared” for growth and tissue healing).
Foods and fluids containing potassium or phos-phorus (bananas, citrus fruits
and juices, coffee) are restricted. Potassium intake is usually restricted to 40
to 60 mEq/day, and sodium is usually restricted to 2 g/day. The patient may
require parenteral nutrition.
The
oliguric phase of ARF may last 10 to 20 days and is fol-lowed by the diuretic
phase, at which time urine output begins to increase, signaling that kidney
function is returning. Blood chem-istry evaluations are made to determine the
amounts of sodium, potassium, and water needed for replacement, along with
assess-ment for overhydration or underhydration. After the diuretic phase, the
patient is placed on a high-protein, high-calorie diet and is encouraged to
resume activities gradually.
Nursing Management
The
nurse has an important role in caring for the patient with ARF. In addition to
directing attention to the patient’s primary disorder (which may be a factor in
the development of ARF), the nurse monitors for complications, participates in
emergency treatment of fluid and electrolyte imbalances, assesses progress and
response to treatment, and provides physical and emotional support. Additionally,
the nurse keeps family members informed about the patient’s condition, helps
them understand the treat-ments, and provides psychological support. Although
the devel-opment of ARF may be the most serious problem, the nurse must
continue to include in the plan of care those nursing measures in-dicated for
the primary disorder (eg, burns, shock, trauma, ob-struction of the urinary
tract).
MONITORING FLUID AND ELECTROLYTE BALANCE
Because
of the serious fluid and electrolyte imbalances that can occur with ARF, the
nurse monitors the patient’s serum electrolyte levels and physical indicators
of these complications during all phases of the disorder. Hyperkalemia is the
most immediate life-threatening imbalance seen in ARF. Parenteral fluids, all
oral in-take, and all medications are screened carefully to ensure that hidden
sources of potassium are not inadvertently administered or consumed.
Intravenous solutions must be carefully selected ac-cording to the patient’s
fluid and electrolyte status. The patient’s cardiac function and
musculoskeletal status are monitored closely for signs of hyperkalemia.
The
nurse monitors fluid status by paying careful attention to fluid intake
(intravenous medications should be administered in the smallest volume
possible), urine output, apparent edema, distention of the jugular veins,
alterations in heart sounds and breath sounds, and increasing difficulty in
breathing. Accurate daily weights, as well as intake and output records, are
essential.
Indicators
of deteriorating fluid and electrolyte status are re-ported immediately to the
physician, and preparation is made for emergency treatment. Hyperkalemia is
treated with glucose and insulin, calcium gluconate, cation-exchange resins
(Kayexalate), or dialysis. Fluid and other electrolyte disturbances are often
treated with hemodialysis, peritoneal dialysis, or other continu-ous renal
replacement therapies.
REDUCING METABOLIC RATE
The
nurse also directs attention to reducing the patient’s meta-bolic rate during
the acute stage of renal failure to reduce catab-olism and the subsequent
release of potassium and accumulation of endogenous waste products (urea and
creatinine). Bed rest may be indicated to reduce exertion and the metabolic
rate during the most acute stage of the disorder. Fever and infection, both of
which increase the metabolic rate and catabolism, are prevented or treated
promptly.
PROMOTING PULMONARY FUNCTION
Attention
is given to pulmonary function, and the patient is as-sisted to turn, cough,
and take deep breaths frequently to prevent atelectasis and respiratory tract
infection. Drowsiness and lethargy may prevent the patient from moving and
turning without en-couragement and assistance.
PREVENTING INFECTION
Asepsis
is essential with invasive lines and catheters to minimize the risk of
infection and increased metabolism. An indwelling uri-nary catheter is avoided
whenever possible because of the high risk for UTI associated with its use.
PROVIDING SKIN CARE
The
skin may be dry or susceptible to breakdown as a result of edema; therefore,
meticulous skin care is important. Additionally, excoriation and itching of the
skin may result from the deposit of irritating toxins in the patient’s tissues.
Massaging bony promi-nences, turning the patient frequently, and bathing the
patient with cool water are often comforting and prevent skin breakdown.
PROVIDING SUPPORT
The
patient with ARF requires treatment with hemodialysis, peri-toneal dialysis, or
continuous renal replacement therapies to pre-vent serious complications; the
length of time that these treatments are necessary varies with the cause and
extent of damage to the kidneys. The patient and family need assistance,
explanation, and support during this time. The purpose and ra-tionale of the
treatments are explained to the patient and family by the physician. High
levels of anxiety and fear, however, may necessitate repeated explanation and
clarification by the nurse. The family members may initially be afraid to touch
and talk to the patient during the procedure but should be encouraged and
assisted to do so.
Although
many of the nurse’s functions are devoted to the technical aspects of the
procedure, the psychological needs and concerns of the patient and family
cannot be ignored. Continued assessment of the patient for complications of ARF
and of its pre-cipitating cause is essential.
Related Topics