Chapter: Microbiology
Antibodies
ANTIBODIES
Antibodies are glycoprotein molecules which are produced in response to an antigen, and reacts specifically with it in an observable manner
· Tiselius in 1937 analyzed serum by free zone electrophoresis and characterized proteins at pH 8.6
All proteins have negative charge and move towards anode
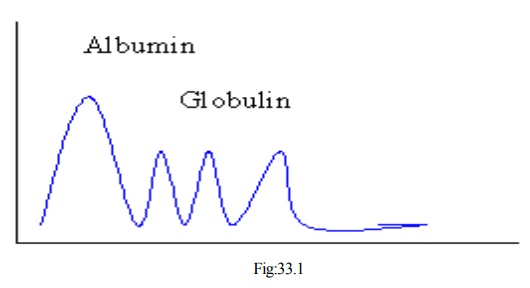
§ Tiselius and Kabat analyzed rabbit’s hyperimmune sera before and after absorption with immunizing agent
§ After absorption there was pronounced decrease in γ globulin
§ Hence antibody activity was traced down to γ globulin
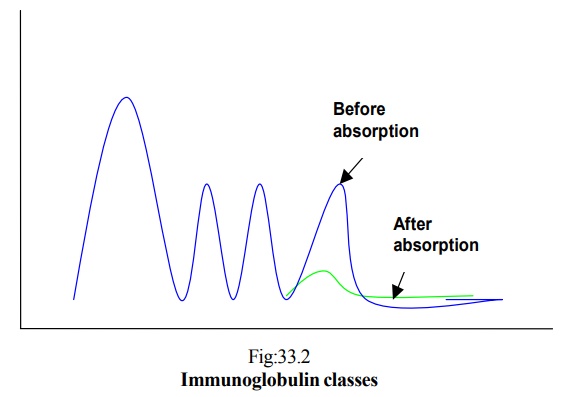
· γ globulin is not a homogeneous protein
· In 1964 WHO international agreement selected the generic name Immunoglobulin for all antibody containing proteins
· They subdivided immunoglobulin into different classes
Immunoglobulin classes
· In man 5 major classes of Ig are described
- Ig G : The major serum component
- Ig M : Macroglobulin
- Ig A : Present predominantly in secretions
- Ig D : Important cell membrane receptor form
- SgE : Antibody involved in hypersensitivity reac-209
Characterization of antibodies
· Early physical – chemical studies were done with Ig G from horse, rabbit and human
· Important structural features were predicted even before sophisti-cated studies were available
· Molecular weight was calculated from sedimentation and diffusion studies
· Asymmetrical and or non globular form by viscosity studies
· Globular domain structure from unique susceptibility to proteolytic enzymes
· Two antigen binding sites by hapten antibody reactions
· Thus early studies predicted three functional domains and have been confirmed
Structure of antibodies
· Before going into the structure of antibody, one must know the structure of proteins
· Proteins are made from amino acids
· Amino acids form poly peptide chains
· Polypeptides form proteins
· Proteins have 3 dimensional structure
o If any change in the primary sequence of amino acid in polypeptide
o Or in three dimensional structure there is change in the property
Digestion with enzymes
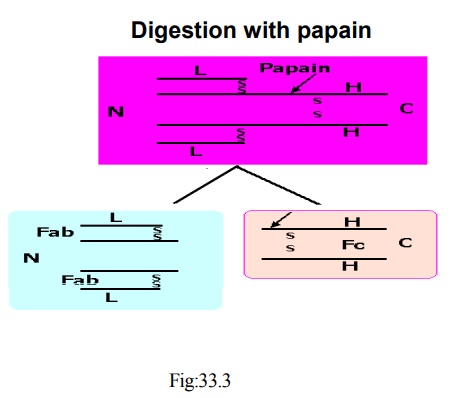
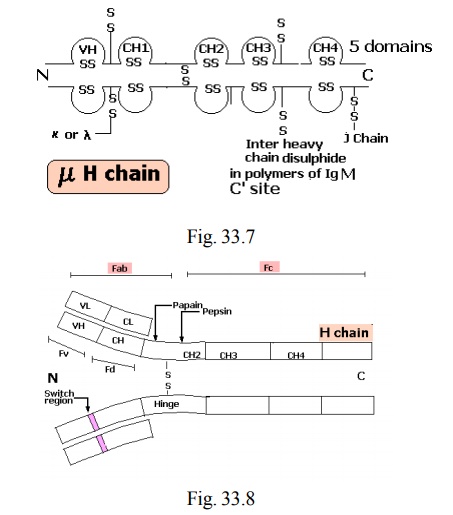
· Rodney and Porter digested rabbit Ig with the enzyme papain
· It cleaved the molecule and produced two major fractions and a small amount of short peptides
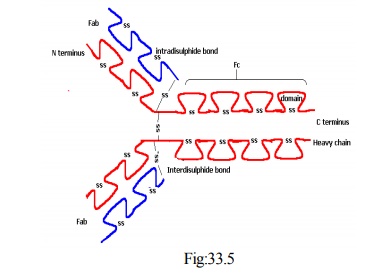
· One fraction (MW 45,000) still possessed antigen binding site and was named as fragment antibody binding (Fab)
· The other fragment could be crystallized, and was called Fragment crystallized (Fc)
· Fab possessed antigen binding site but was monovalent
· Possessed one reactive site
· Could not cross link antigen molecule
· When one added up the molecular weights of Fab and Fc frag-ments, plus the observation that the Fab was monovalent, it ap-peared that the original antibody contained:
· Two Fab fragments and one Fc fragment
General formula for antibody
· The general formula for antibody is (H2 L2)n
· The immunoglobulins are made of 2 heavy chains and 2 light chains
· These are held together by covalent bonds
· These bonds are interchain disulphide bridges
· Each chain is made of a number of loops
· These loops are known as domains
· Each domain is formed by intrachain disulphide bonds
· There are 2 loop sections per L chain and 4 loop sections per H chain
· There are two terminals in each chain
· One is called C terminus
· Other is called N terminus
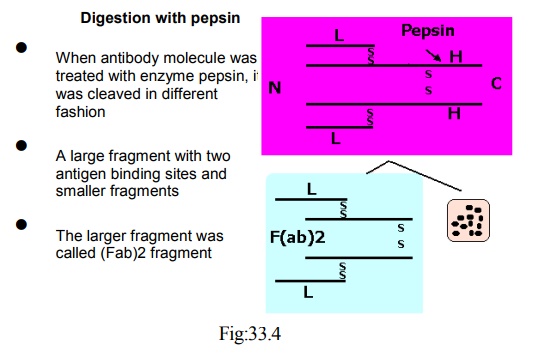
Digestion with pepsin
· When antibody molecule was treated with enzyme pepsin, it was cleaved in different fashion
· A large fragment with two antigen binding sites and smaller fragments
· The larger fragment was called (Fab)2 fragment
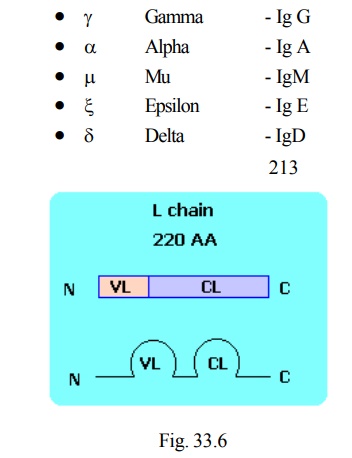
Light chain (Fig 33.6)
· C terminus contains the constant region
· N terminus contains the variable region
· L chain is named as Kappa (k) and lambda (λ )
· contains two domains
· domain at N terminus is variable domain of light chain calledVL
· domain at C terminus is constant domain called CL
Types of heavy chain
· There are 5 different types of H chains
· Based on the type of H chain the classes of antibody is determined
They are:
Properties and functions of immunoglobulins
Ig M
· Ig M is the main immunoglobulin produced early in primary im-mune response
· It is present on the surface of all uncommitted B lymphocytes
· IgM is a pentamer and the valence is 10
· Ig M is the most efficient immunoglobulin in agglutination, comple-ment fixation and other antigen antibody reactions
· It plays an important role in the defense against bacterial and viral diseases
· It does not cross placenta
Ig G
· Ig
· G has two identical antigen binding sites and is bivalent
· There are four subclasses namely Ig G1,Ig G2, IgG3 and IgG4
· Ig G is the predominant antibody in secondary immune response
· It plays an important role in defense against bacteria, viruses
· It also neutralizes toxins
· It crosses placenta and is found in large quantities in newborns
Ig A
1. Ig A is found mainly in secretions like milk, tears, saliva and secre-tions of respiratory, intestinal and genital tracts
2. It protects the mucus membranes against microbial attack
3. As many microbes enter the body through these mucus membranes, Ig A offers the first line of defense
4. Each IgA molecule consists of two H2L2 units and a J chain and a secretory component.
5. The secretory component is a polypeptide synthesized by epithe-lial cells and it helps IgA to pass the mucosal surface
IgE
· IgE antibody is present in increased quantities in allergic individu-als\
· The Fc portions of the molecule binds to mast cells and eosinophils
· When this antibody combines with its antigen on the mast cell sur-face, it leads to allergic response
IgD
· IgD has no antibody function
· It may act as antigen receptor on cells
· In serum it is present in only trace amounts
Related Topics