Chapter: Essential Microbiology: Biochemical Principles
Acids, bases, and pH
Acids, bases,
and pH
Only a minute proportion of water molecules, something like one
in every 5 × 108, is present in
its dissociated form, but as we have already seen, the H+ and OH− ions play an important part in
cellular reactions. A solution becomes acid or alkaline if there is an
imbalance in the amount of these ions present. If there is an excess of H+ , the solution becomes acid, whilst if OH− predominates, it becomes alkaline. The pH of a solution is an expression of the molar concentration of
hydrogen ions:
pH= −log10[H+ ]
In pure water, hydrogen ions are present at a concentration of
10−7M, thus the pH is 7.0. This is
called neutrality, where the solution is neither acid or alkaline. At higher
concentrations of H+ , such as 10−3M (1 millimolar), the pH value is lower, in this case
3.0, so acid solutions have a value below 7. Conversely, alkaline solutions
have a pH above 7. You will see from this example that an increase of 104
(10 000)-fold in the [H+ ] leads to a change of only four points on the pH scale. This
is because it is a logarithmic scale; thus a solution of pH 10 is 10 times more
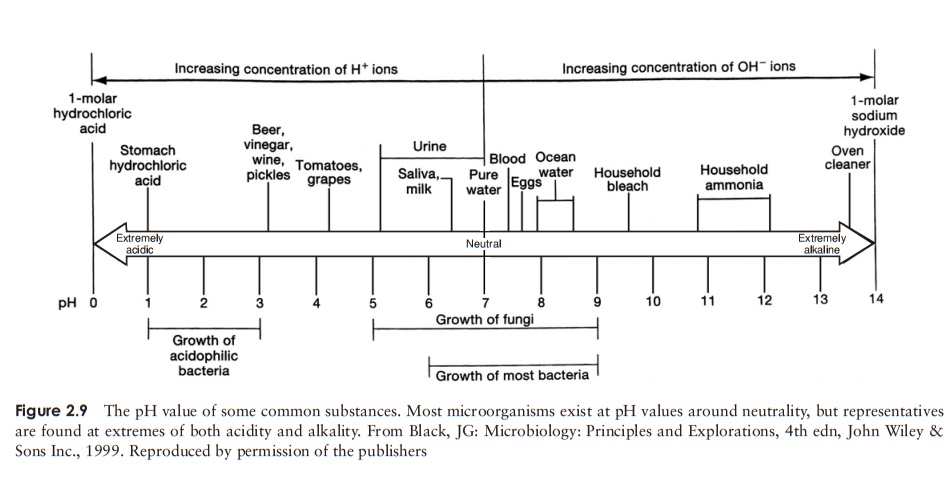
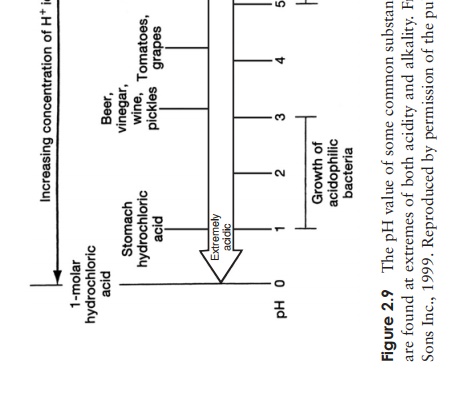
alkaline than one of pH 9, and 100 times more than one of pH 8. Figure 2.9 shows
the pH value of a number of familiar substances.
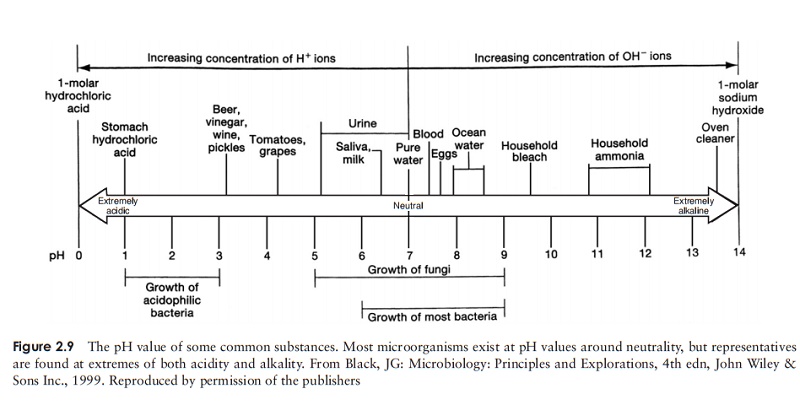
Most microorganisms live in an aqueous environment, and the pH
of this is very important. Most will only tolerate a small range of pH, and the
majority occupy a range around neutrality, although as we shall see later on in
this book, there are some startling
exceptions to this. Most of the important molecules involved in the chemistry
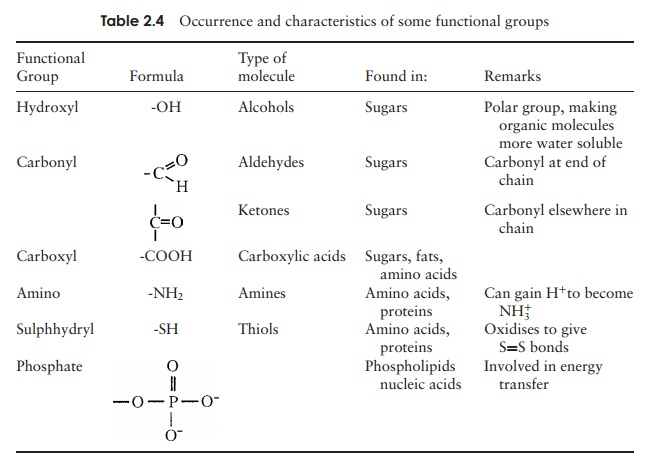
of living cells are organic, that is, they are based on a skeleton of
covalently linked carbon atoms. Biological molecules have one or more functional groups attached to this
skeleton; these are groupings of atoms with distinctive reactive properties,
and are responsible for many of the chemical properties of the organic
molecule. The possession of a functional group(s) frequently makes an organic
molecule more polar and therefore more soluble in water.
Some of the most common functional groups are shown in Table
2.4. It can be seen that the functional groups occur in simpler organic
molecules as well as in the macromolecules we consider below.
Related Topics