Chapter: Biochemical Pharmacology : G protein-coupled receptors
Structure and function of the nicotinic acetylcholine receptor
Structure and function of the
nicotinic acetylcholine receptor
The nicotinic acetylcholine
receptor (NAR) is the most widely studied receptor ion channel. This is due to
a very practical reason – availability. The receptor can be isolated in high
yield from electric eel or electric ray, both of which use strong electric
discharges to incapacitate their prey or for defence. In the electric organs of
these fish, the receptor occurs in abundance in stacks of excitable cells
(Figure 9.2). Importantly, however, the NAR and voltage-gated ion channels only
occur on one side of the cell.
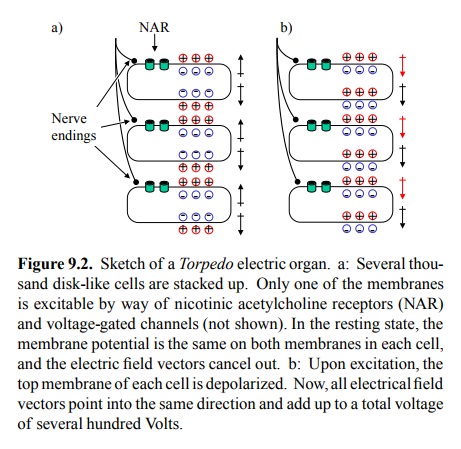
In the
resting state, both sides of the cell will have the same membrane potential. As
a consequence, the electric field vectors within the cell and within the entire
stack will can-cel each other out (Figure 9.2a). Electric stimulation will
depolarize one membrane in each cell and invert its electri-cal field. Now, all
of a sudden, all field vectors in the entire stack will point into the same
direction (Figure 9.2b), and thus will add up to one very strong electrical
field. Each in-dividual cell will contribute about 150 mV (the potential of its
two membranes connected in series). Since the overall voltage is about 500-1000
V, this obviously requires sever-al thousands of cells stacked on top of each
other. More-over, since both voltage and current are needed to make an impact
on the prey, each level within the stack will need to have a sizeable surface
area, thus providing for a large num-bers of NAR molecules.
Overall structure
A
variety of experimental approaches have been taken to study the structure of
NAR. A very interesting one is `elec-tron crystallography', performed with NAR
incorporated into artificial lipid membranes (liposomes) and there form-ing
regularly packed arrays (Figure 9.3a, right). Electrons (not X rays, which are
normally used in protein crystallog-raphy) scattered from these two-dimensional
crystals will form a pattern that can be evaluated to yield a three-dimen
sional map of the structure, provided that data are avail-able from various
tilt angles of the crystals relative to the electron beam. The resolution of
structural images thus ob-tained is not quite the same as that of X-ray
crystallography. However, X-ray data are often very hard to obtain with
inte-gral membrane proteins; in fact, in the structures available, the
transmembrane parts are often missing2.
Figure 9.3b shows `contour' maps of the NAR, in
top and in side view. In the top view, the identities of the individu-al
subunits are also indicated; these have been determined using samples labelled
with antibody fragments specific for each subunits. Note that the subunit
composition of the NAR varies between different tissues. The α2βγδ pat-tern shown here holds for the fish
electric organs 3, and for the muscle type NAR in humans. Neuronal
NAR, includ-ing the one found in the autonomic ganglia, is α2β3 or α3β2. This structural difference accounts for the selective action of
several agonistic and antagonic drugs on muscles or the ganglia, respectively
(see below).
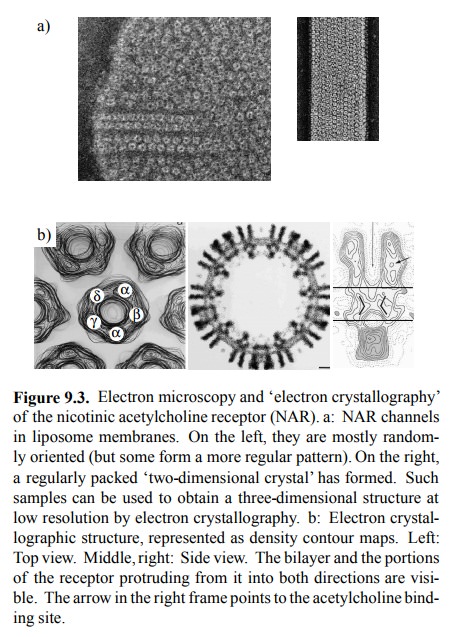
Figure 9.3. Electron microscopy and `electron crystallography' of the nicotinic acetylcholine receptor (NAR). a: NAR channels in liposome membranes. On the left, they are mostly random-ly oriented (but some form a more regular pattern). On the right, a regularly packed `two-dimensional crystal' has formed. Such samples can be used to obtain a three-dimensional structure at low resolution by electron crystallography. b: Electron crystal-lographic structure, represented as density contour maps. Left: Top view. Middle, right: Side view. The bilayer and the portions of the receptor protruding from it into both directions are visi-ble. The arrow in the right frame points to the acetylcholine bind-ing site.
In the
side view, it can be seen that a significant part of the receptor protrudes
from the membrane to the exterior. The `bottleneck' or gate is located at the
level of the lipid bilayer. An additional portion of the receptor sits on the
cytosolic surface of the receptor and may be important for its ion selectivity.
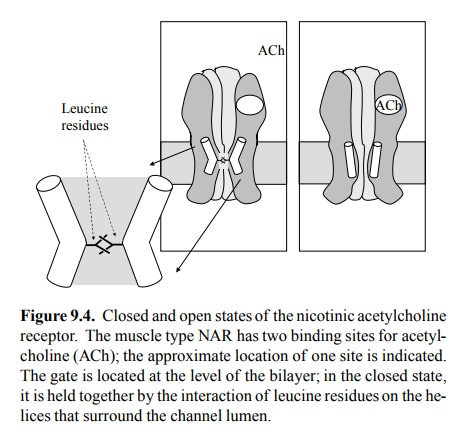
The
bottleneck is lined by 5 α-helices (one each from the 5 subunits). In the
closed state, they touch upon each oth-er, making direct hydrophobic contacts
via some leucine residues (Figure 9.4). These helices move apart by a
con-siderable distance during channel opening, creating a rather large free
lumen.
Location of the acetylcholine binding site
The acetylcholine binding site was mapped onto
the struc-ture by comparing the electron densities of ligand-bound and unbound
samples. This site has also been extensively characterized with biochemical
methods, which allow the assignment of amino acid residues involved in ligand
bind-ing. One important technique consists in affinity labelling. Figure 9.5
summarizes one such study.
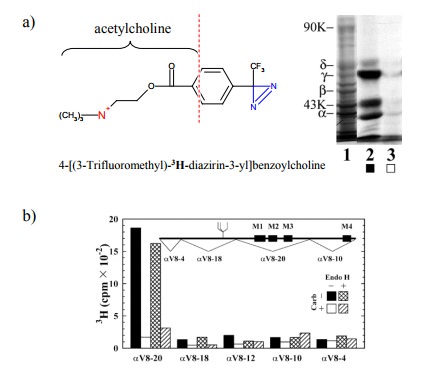
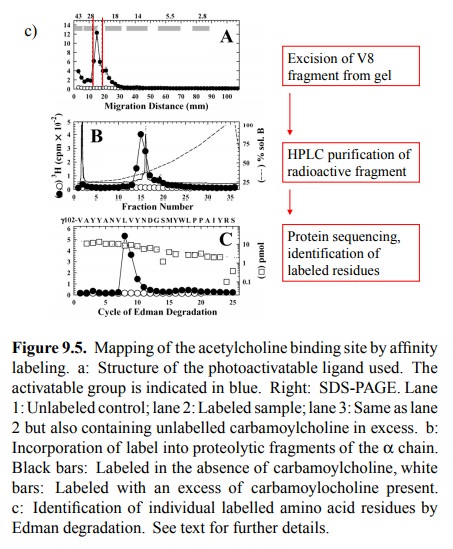
The
compound used is a derivative of acetylcholine. It con-tains tritium (3H)
and thus is radioactive; it also contains a photo-activatable reactive group
(blue). If allowed to bind to the receptor and illuminated with UV light, this
group will attach itself onto anything in the vicinity, including amino acid
residues, even ones of very low intrinsic reac-tivity. This will lead to the
incorporation of the radioactive label into the receptor. The gel shows the
labelled α and γ subunits (as well as a third band that
reportedly is a degra-dation product of the γ chain). Importantly, this labeling oc-curs
selectively at the agonist binding site, since it is large-ly suppressed in the
presence of excess carbachol, which is a synthetic agonist closely related to
acetylcholine (see later).
After labelling, the chains
were separated4 and individually cleaved with a site-specifically
acting protease (V8) 5. Fig-ure 9.5b shows that, in the α chain, the bulk of the radioac-tivity is associated with one of
the proteolytic fragments (named αV8-20). However, specific labelling (i.e.,
label-ing that can be suppressed by the specific competitor car-bamoylcholine)
also occurs in other fragments.
Individual proteolytic
fragments, in turn, were isolated by electrophoresis and further purified by
HPLC, and the la-belled residues within them were identified by protein
se-quencing (Figure 9.5c). With the fragment shown, most of the label is found
attached to two adjacent resides (Leu109 and Val 110).
While
with the reagent used here only the α and the γ chains were strongly labelled, previous
experiments (using different affinity probes with reactive groups attached to
different sites of the acetylcholine molecule) have provided evidence that the δ chain is involved in ligand binding as well. In fact, there are
two binding sites for acetylcholine in muscle-type NAR, located at the
interfaces of the two α chains with the γ and the δ chains, respectively (cf. Fig-ure 9.3b).
The nature of the receptor-ligand interaction
Virtually
all agonists and antagonists at the NAR share the positive charge of
acetylcholine, most commonly (as in acetylcholine) in the form of a quaternary
amino group6. What role does this positive charge have? The agonist
bind-ing site of NAR is not rich in negative charges (aspartate or glutamate
residues) but instead has multiple aromatic side chains. These can bind to
cations by a peculiar mech-anism, called the `cation-π' interaction, in which the fixed charge of the cation is
accommodated by the mobile π elec-trons of the aromatic ring. Using a quite
sophisticated set of methods, it was determined that this mechanism indeed is
responsible for the binding of acetylcholine to the NAR. These experiments were
carried out as follows (Figure 9.6):
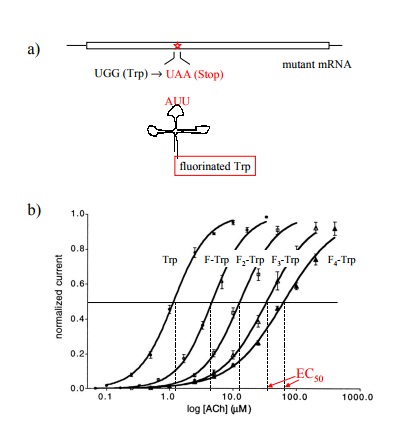
1. The codons of individual tryptophan residues in
the cloned α chain were exchanged for an amber stop codon (TAA) and the mutant
mRNA was obtained by in vitro
transcription.
2. A suppressor tRNA – a tRNA carrying a mutant
anti-codon that recognizes a complementary stop codon – was likewise generated in vitro and synthetically acy-lated
with various fluorinated derivatives of tryptophan. This tRNA will selectively
incorporate its amino acyl cargo at the mutant stop codon.
3. The mRNA and the tRNA were both injected into
frog oocytes.
4. The frog oocytes expressing the mutant channels
were studied using the `patch-clamp' method, which allows the characterization
of single channels on intact cells7.
The non-natural tryptophan derivatives
incorporated at the mutant stop codon contained various numbers of fluorine as
substituents at the benzene ring that is part of the in-dole in the tryptophan
side chain (cf. Figure 9.6c). In Fig-ure 9.6b, it is shown that with increasing
numbers of fluo-rine substituents the sensitivity of the receptor to activation
by acetylcholine decreased continuously, as ever higher amounts of the agonist
were required to achieve half-max-imal response.
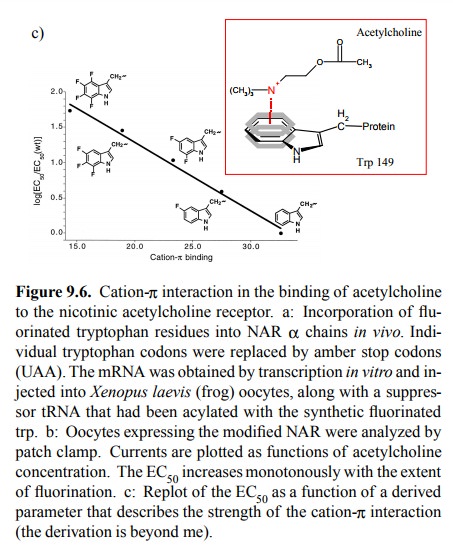
Fluorine is very small and is
considered not to cause major steric changes when substituted for hydrogen.
However, it is very strongly electronegative and will therefore pull π electrons out of the ring; this will weaken the cation-π inter-action. Figure 9.6c plots the observed receptor
sensitivities (as logarithms of the EC508) against a
theoretical parameter that describes the intensity of the cation-π interaction for tryptophan and its fluorinated derivatives. The
correlation suggests that this interaction indeed is very important for the
binding of acetylcholine to the NAR. By comparing the effects of substituting
different tryptophan residiues, it was also determined that the most
significant single residue in this interaction is tryptophan 149 in the α chain.
Receptor desensitization
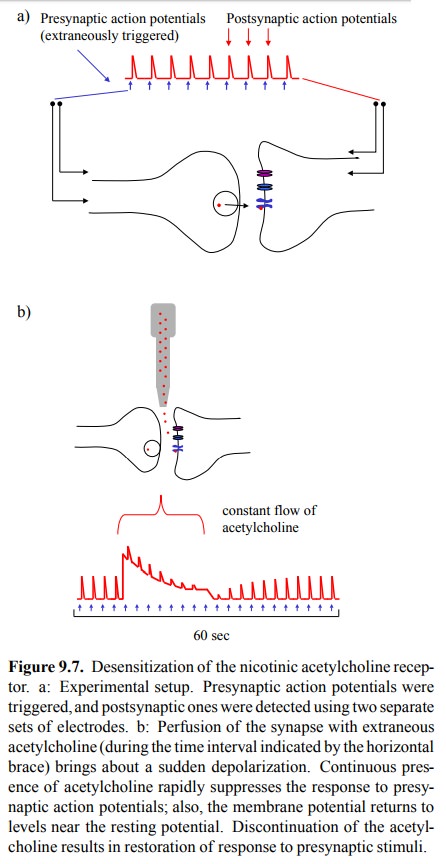
An aspect of NAR function
that is very important in its pharmacological manipulation is receptor
desensitization. This phenomenon can be experimentally demonstrated in the
set-up depicted in Figure 9.7: Stimulating electrodes produce presynaptic
action potentials, which induce release of acetylcholine into the synaptic
cleft. Acetylcholine will trigger postsynaptic action potentials, which in turn
are detected with a second pair of electrodes. These postsynap-tic action
potentials attain a reproducible, fairly constant height, and they are of very
short duration, which reflects swift removal of acetylcholine by
cholinesterase. If extra-neous acetylcholine is applied to this system (in a
contin-uous fashion, so as to keep up a constant level in spite of
cholinesterase activity), there is a steep initial depolariza-tion of the
membrane, which then peters out over a time interval of a few seconds.
Additional presynaptic stimuli during this time period are ineffectual. If the
extrinsic sup-ply of acetylcholine is discontinued, cholinesterase rapid-ly
cleans up, the membrane completely repolarizes, and its sensitivity to
presynaptic triggers is restored in another few seconds.
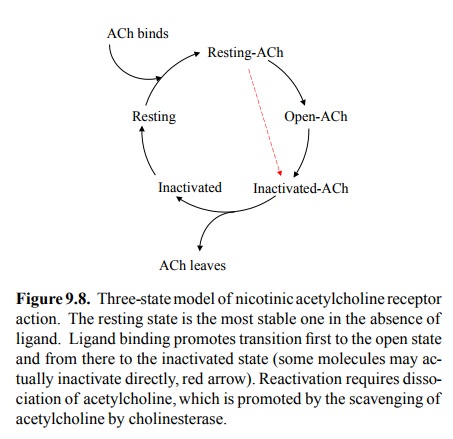
Receptor desensitization can
be understood in terms of a model of channel function that is similar to what
we have seen before with voltage-gated channels (Figure 9.8). The receptor can
cycle between three functional states: Resting (no current), open (current),
and inactivated (no current). In the absence of ligand, the resting state is
the most stable one, so most receptors will be in this state. Ligand binding
favours both the open and the inactivated states over the resting state.
Therefore, at sufficiently high ligand concen-tration, essentially all receptor
molecules will leave the rest-ing state. Importantly, the open state is
kinetically favoured over the inactivated state, which is a fancy way of saying
that opening is faster than inactivation. Thus, most chan-nels will initially
enter the open state (although some will become directly inactivated). However,
the inactivated state is thermodynamically favoured over the open state, which
means it is more stable than the latter. In fact, at high con-centrations of
ligand, it is the most stable of all three states, and therefore upon
continuous exposure to acetylcholine all the receptors that initially opened
eventually wind up in the inactivated state. This explains the above
observation of membrane repolarization and insensitivity during perfusion of
the synapse with acetylcholine.
Related Topics