Chapter: Biochemistry: Photosynthesis
Photosystems I and II and the Light Reactions of Photosynthesis
Photosystems I and II and the
Light Reactions of Photosynthesis
In the light
reactions of photosynthesis, water is converted to oxygen by oxidation and NADP+
is reduced to NADPH. The series of redox reactions is coupled to the
phosphorylation of ADP to ATP in a process called photophosphorylation.
H2O
+ NADP+ - > NADPH + H+ + O2
ADP + Pi
- > ATP
The
light reactions consist of two parts, accomplished by two distinct but related
photosystems. One part of the reaction is the reduction of NADP+ to
NADPH, carried out by photosystem I
(PSI). The second part of the reaction is the oxidation of water to produce
oxygen, carried out by photosystem II
(PSII). Both photosystems carry out redox (electron transfer) reactions.
The two photosystems interact with each other indirectly through an electron
transport chain that links the two photosystems. The production of ATP is
linked to electron transport in a process similar to that seen in the
production of ATP by mitochondrial electron transport.
In the
dark reactions, the ATP and NADPH produced in the light reaction provide the
energy and reducing power for the fixation of CO2. The dark
reac-tions also constitute a redox process, since the carbon in carbohydrates
is in a more reduced state than the highly oxidized carbon in CO2.
The light and dark reactions do not take place separately, but they are
separated for purposes of discussion only.
The net
electron transport reaction of the two photosystems taken together is, except
for the substitution of NADPH for NADH, the reverse of mitochon-drial electron
transport. The half-reaction of reduction is that of NADP+ to NADPH,
whereas the half-reaction of oxidation is that of water to oxygen.
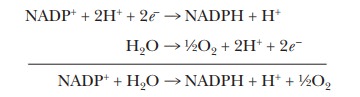
This is
an endergonic reaction with a positive ∆G°
= +220 kJ mol-1 = +52.6 kcal mol-1. The light energy
absorbed by the chlorophylls in both photosystems provides the energy that
allows this endergonic reaction to take place. A series of electron carriers
embedded in the thylakoid membrane link these reactions. The electron carriers
have an organization very similar to the carriers in the electron transport
chain.
Photosystem
I can be excited by light of wavelengths shorter than 700 nm, but photosystem
II requires light of wavelengths shorter than 680 nm for excitation. Both
photosystems must operate for the chloroplast to produce NADPH, ATP, and O2,
because the two photosystems are connected by the electron transport chain. The
two systems are, however, structurally distinct in the chloroplast; photosystem
I can be released preferentially from the thy-lakoid membrane by treatment with
detergents. The reaction centers of the two photosystems provide different
environments for the unique chlorophylls involved. The unique chlorophyll of
photosystem I is referred to as P700, where P is for pigment and the
subscript 700 is for the longest wavelength of absorbed light (700 nm) that
initiates the reaction. Similarly, the reaction-center chlo-rophyll of
photosystem II is designated P680 because the longest wavelength of
absorbed light that initiates the reaction is 680 nm. Note particularly that
the path of electrons starts with the reactions in photosystem II rather than
in pho-tosystem I. The reason for the nomenclature is that photosystem I was
studied extensively at an earlier date than photosystem II because it is easier
to extract photosystem I from the thylakoid membrane than it is to extract
photosystem
There
are two places in the reaction scheme of the two photosystems where the
absorption of light supplies energy to make endergonic reactions take place
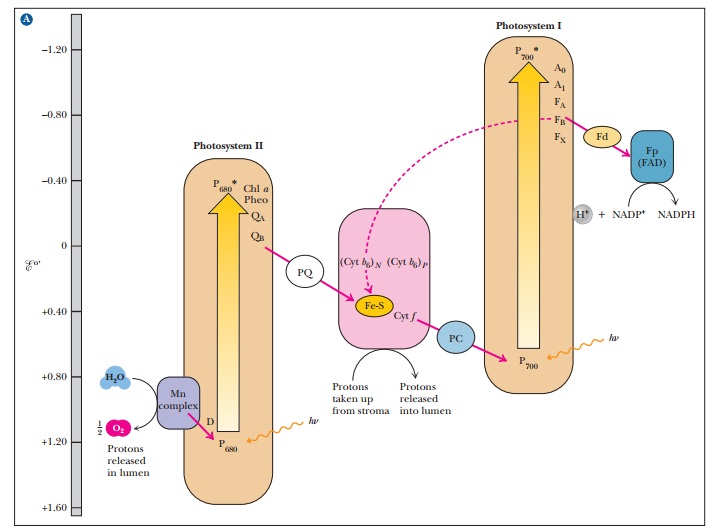
(Figure 22.6).
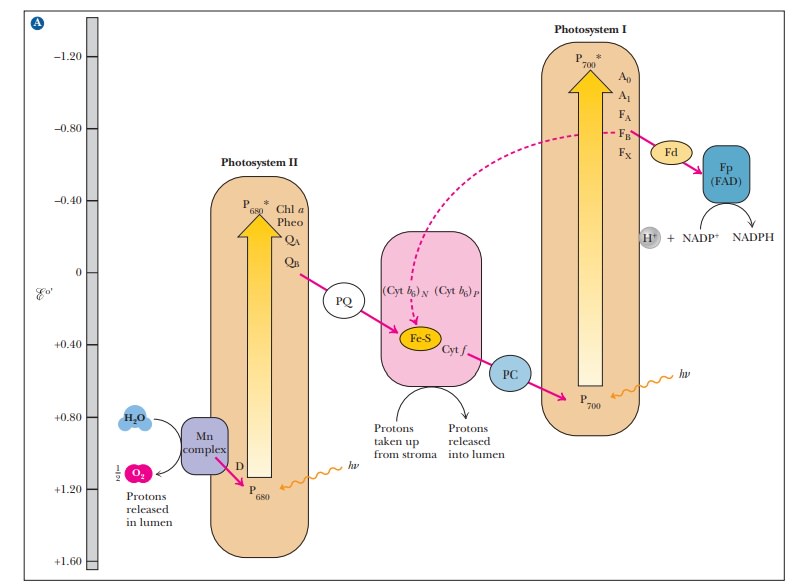
Neither
reaction-center chlorophyll is a strong enough reducing agent to pass electrons
to the next substance in the reaction sequence, but the absorp-tion of light by
the chlorophylls of both photosystems provides enough energy for such reactions
to take place. The absorption of light by Chl (P680) allows
electrons to be passed to the electron transport chain that links photosystem
II and photosystem I and generates an oxidizing agent that is strong enough to
split water, producing oxygen. When Chl (P700) absorbs light, enough
energy is provided to allow the ultimate reduction of NADP+ to take
place. (Note that the energy difference is shown on the vertical axis of Figure
22.6. This type of diagram is also called a Z
scheme. The Z is rather lopsided and lies on its side, but the name is
common.) In both photosystems, the result of supplying energy (light) is
analogous to pumping water uphill.
How does photosystem II split water to produce oxygen?
The
oxidation of water by photosystem II to produce oxygen is the ultimate source
of electrons in photosynthesis. These electrons are subsequently passed from
photosystem II to photosystem I by the electron transport chain. The electrons
from water are needed to ÒÞll the holeÓ that is left when the absorption of one
photon of light leads to donation of an electron from photosystem II to the
electron transport chain.
The
electrons released by the oxidation of water are first passed to P680,
which is reduced. There are intermediate steps in this reaction because four
electrons are required for the oxidation of water, and P680* can
accept only one electron at a time. A manganese-containing protein complex and
several other protein components are required. The oxygen-evolving complex of photosys-tem II passes through a series
of five oxidation states (designated as S0 through S4) in
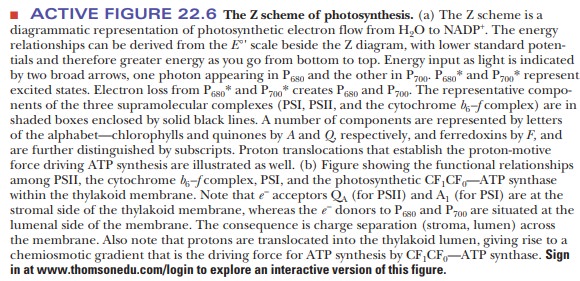
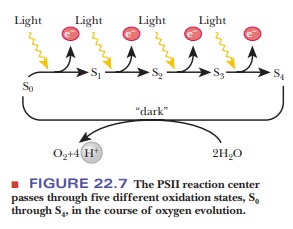
the transfer of four electrons in the process of evolving oxygen (Figure 22.7).
One electron is passed from water to PSII for each quantum of light. In the
process, the components of the reaction center go successively through
oxidation states S1 through S4. The S4 decays
spontaneously to the S0 state and, in the process, oxidizes two
water molecules to one oxygen molecule. Note that four protons are released
simultaneously. The immediate electron donor to the P680
chlorophyll, shown as D in Figure 22.6, is a tyrosine residue of one of the
protein components that does not contain manganese. Several quinones serve as
intermediate electron transfer agents to accommodate four electrons donated by
one water molecule. Redox reactions of manganese also play a role here. Even
this mechanism is an oversimplification. Attempts to observe the direct
pro-duction of oxygen by the S4 state imply that some intermediate
(S4') directly produces oxygen after deprotonation of S3
and loss of an electron by S4. The main point is that the
oxygen-evolving complex is very complex indeed.
In
photosystem II, as in photosystem I, the absorption of light by chlo-rophyll in
the reaction center produces an excited state of chlorophyll. The wavelength of
light is 680 nm; the reaction-center chlorophyll of photosystem
is also
referred to as P680. The excited chlorophyll passes an electron to a
pri-mary acceptor. In photosystem II, the primary electron acceptor is a
molecule of pheophytin (Pheo), one
of the accessory pigments of the photosynthetic apparatus. The structure of
pheophytin differs from that of chlorophyll only in the substitution of two
hydrogens for the magnesium. The transfer of electrons is mediated by events
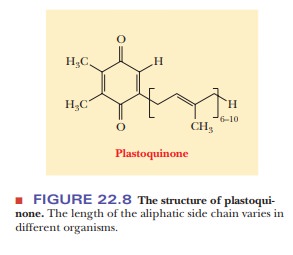
that take place at the reaction center. The next electron acceptor is plastoquinone (PQ). The structure of
plastoquinone (Figure 22.8) is similar to that of coenzyme Q (ubiquinone), a
part of the respiratory electron transport chain, and plastoquinone serves a
very similar purpose in the transfer of electrons and hydrogen ions.
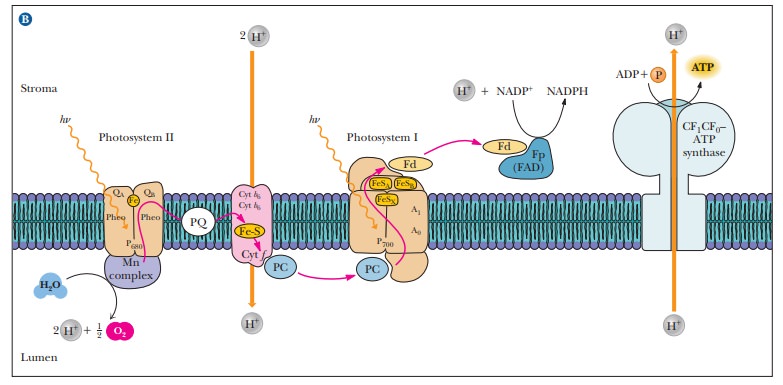
The
electron transport chain that links the two photosystems consists of
pheo-phytin, plastoquinone, a complex of plant cytochromes (the b6–f complex), a copper-containing protein called plastocyanin (PC), and the oxidized
form of P700 (see Figure 22.6). The b6–f complex
of plant cytochromes consists of two b -
type cytochromes (cytochrome b6)
and a c - type cytochrome (cytochromef ).This complex is similar in structure
to the bc1 complex in
mitochondria and occupies a similar central position in an electron transport
chain. This part of the photosynthetic apparatus is the subject of active
research. There is a pos-sibility that a Q cycle may operate here as well, and
the object of some of this research is to establish definitely whether this is
so. In plastocyanin, the copper ion is the actual electron carrier; the copper
ion exists as Cu(II) and Cu(I) in the oxidized and reduced forms, respectively.
This electron transport chain has another similarity to that in mitochondria,
that of coupling to ATP generation.
When the oxidized chlorophyll of P700 accepts electrons from the electron transport chain, it is reduced and subsequently passes an electron to photosys-tem I, which absorbs a second photon of light. Absorption of light by photo-system II does not raise the electrons to a high enough energy level to reduce NADP+; the second photon absorbed by photosystem I provides the needed energy. This difference in energy makes the Z of the Z scheme thoroughly lopsided, but the transfer of electrons is complete.
How does photosystem I reduce
NADP+?
The
absorption of light by P700 then leads to the series of electron
transfer reactions of photosystem I. The substance to which the excited-state
chlorophyll, P700*, gives an electron is apparently a molecule of
chlorophyll a; this transfer of
electrons is mediated by processes that take place in the reaction center. The
next electron acceptor in the series is bound ferredoxin, an iron-sulfur
protein occurring in the membrane in photosystem I. The bound ferredoxin passes
its electron to a molecule of soluble ferredoxin. Soluble ferredoxin in turn
reduces an FAD-containing enzyme called ferredoxin-NADP+ reductase.
The FAD portion of the enzyme reduces NADP+ to NADPH (Figure 22.6).
We can summarize the main features of the process in two equations, in which
the notation ferredoxin refers to the soluble form of the protein.
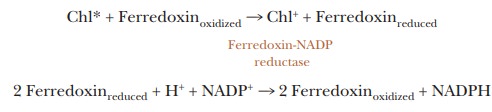
Chl*
donates one electron to ferredoxin, but the electron transfer reactions of FAD
and NADP+ involve two electrons. Thus, an electron from each of two
ferredoxins is required for the production of NADPH.
The net
reaction for the two photosystems together is the flow of electrons from H2O
to NADP+(see Figure 22.6).
2H2O
+ 2NADP+ - > O2 + 2NADPH + 2H+
Related Topics