Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Bohr atom model
Bohr atom model
Neils Bohr in 1913, modified Rutherford's atom
model in order to explain the stability of the atom and the emission of sharp spectral
lines. He proposed the following postulates :
(i) An electron cannot
revolve round the nucleus in all possible orbits. The electrons can revolve
round the nucleus only in those allowed or permissible orbits for which the
angular momentum of the electron is an integral multiple of h/2π (where h
is Planck's constant = 6.626 × 10-34 Js). These orbits are called
stationary orbits or non-radiating orbits and an electron revolving in these
orbits does not radiate any energy.
If m and v are the mass and
velocity of the electron in a permitted orbit of radius r then angular momentum of electron = mvr = nh / 2 π, where n is called principal
quantum number and has the integral values 1,2,3
This is called Bohr's
quantization condition.
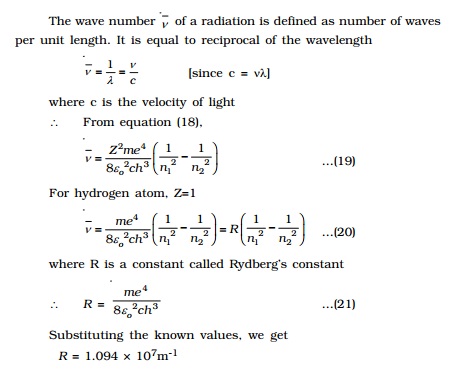
ii) An atom radiates energy, only when an electron jumps from a stationary
orbit of higher energy to an orbit of lower energy. If the electron jumps from
an orbit of energy E2 to an orbit of energy E1, a photon
of energy hν = E2 - E1 is emitted. This condition is
called Bohr's frequency condition.
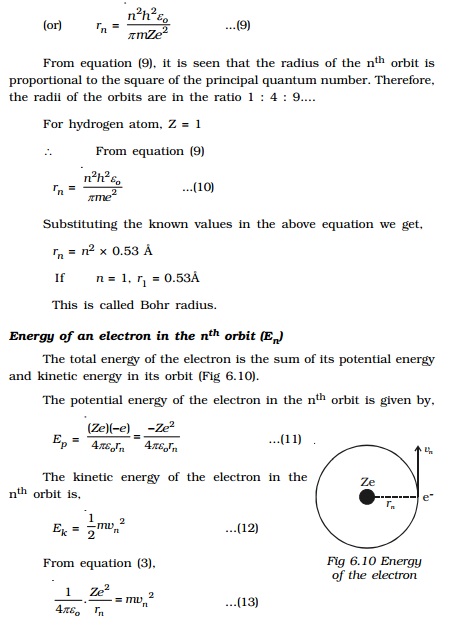
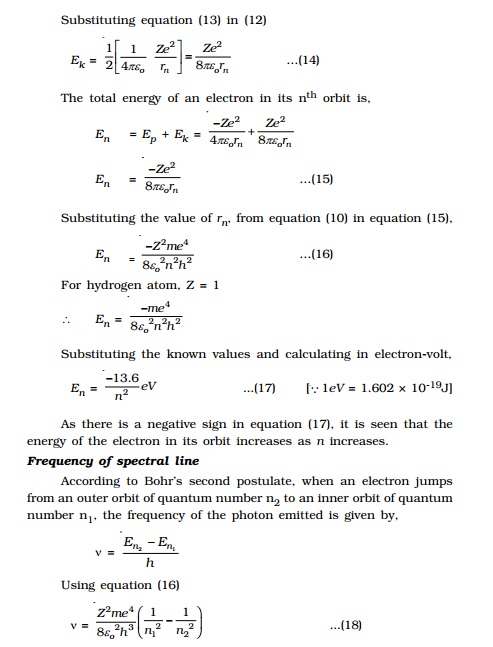
Radius of the nth orbit (rn
)
Consider an atom whose
nucleus has a positive charge Ze,
where Z is the atomic number that
gives the number of protons in the nucleus
and e, the charge of the electron
which is numerically equal to that of proton. Let an electron revolve around
the nucleus in the nth orbit of radius rn.
By Coulomb's law, the
electrostatic force of attraction between the nucleus and the electron = ( 1/ 4πε0 ) . ( Ze)
(e ) / r2n ……. (1)
where εo is the
permittivity of the free space.
Since, the electron revolves
in a circular orbit, it experiences a centripetal force, mv2n / rn = mrnωn2 ………… (2)
where m is the mass of the electron, vn
and ωn are the linear velocity and angular velocity of the electron in
the nth orbit respectively.
The necessary centripetal force is provided by
the electrostatic force of attraction.
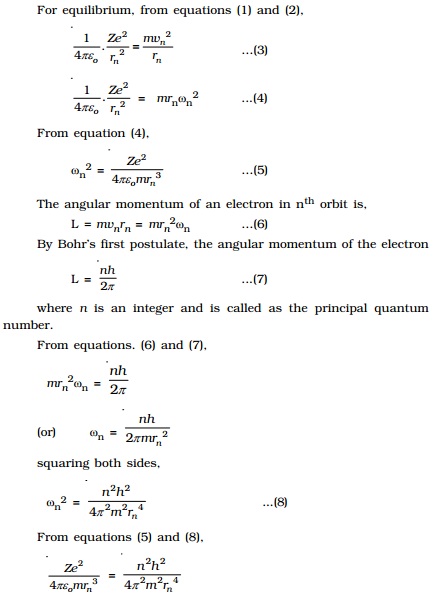
For equilibrium, from equations (1) and (2),
( 1/ 4πε0 ) . ( Ze)
(e ) / r2n = mv2n / rn
Related Topics