Chapter: Biology of Disease: Disorders of the Endocrine System
Thyroid Hormone Disorders
THYROID HORMONE DISORDERS
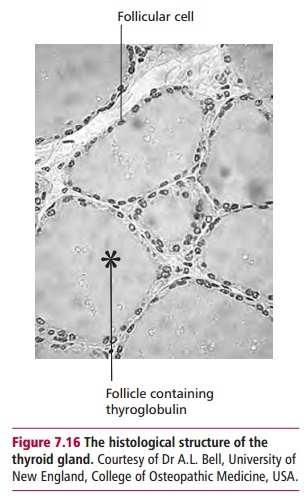
The thyroid gland is the largest endocrine gland in the body, weighing about 20 g. It is a bilobular organ that consists of microscopic spherical follicles with secretory cells (Figure 7.16) that synthesize the thyroid hormones. Thyroid hormones (Figure 7.17 (A) and (B)) consist of triiodothyronine (T3) and thyroxine (T4). Triiodothyronine is the most active, four times that of T4, and has a half-life of 1.5 days compared with 9 days for T4. However in most tissues, particular the liver, T4 can be readily converted to T3. More than 99% of T3 and T4 are transported in the serum as complexes: 70% to thyroxine binding globulin (TBG) and about 20% to albumin and around 10% to prealbumin. The remaining small free portion is the metabolically active fraction.
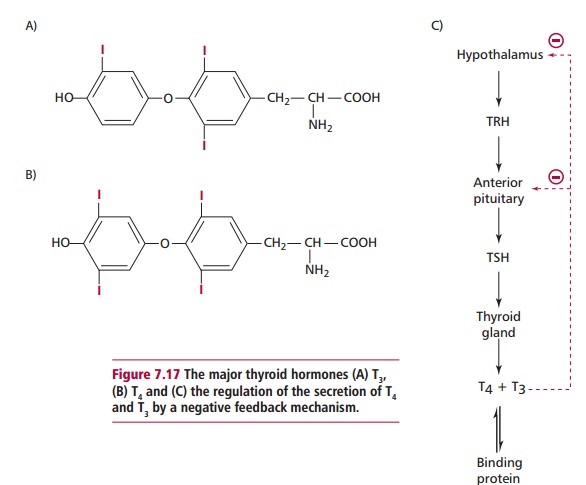
The effects of thyroid hormones are to increase heat production, oxygen consumption, the metabolism of proteins, fats and carbohydrates and to promote normal growth. They are also necessary for the normal functioning of the CNS. Levels of plasma T4 and T3 are regulated by the release of thyroid stimulating hormone (TSH) from the anterior pituitary that, in turn, is controlled by the release of thyrotrophin releasing hormone (TRH) from the hypothalamus. Increasing concentrations of free T4 and T 3inhibit further release of TSH and TRH by a negative feedback effect (Figure 7.17 (C)).
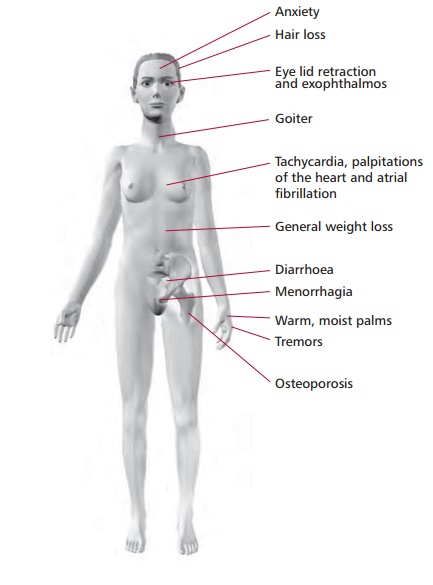
The most serious disorder of thyroid function is hyperthyroidism caused by an excessive production of thyroid hormones. The clinical syndrome resulting from hyperthyroidism is thyrotoxicosis (Figure 7.18). Its clinical features are weight loss, sweating, heat intolerance, anxiety, hyperkinesis, increased appetite, osteoporosis, menorrhagia, tachycardia and pretibial edema.
The commonest cause of hyperthyroidism is Grave’s disease, which can occur at any age but particularly in 20- to 40-year-old females. Patients with Grave’s disease suffer from exophthalmos or protrusion of the eyeballs, in addition to clinical features of hyperthyroidism. It is an autoimmune disease , characterized by the presence of thyroid stimulating antibodies in the blood that bind to TSH receptors in thyroid cells and stimulate them in a similar manner to TSH. Toxic nodules are the second main cause of hyperthyroidism and tend to be found in elderly patients. They may occur singly or as multiples in a nodular goiter and are autonomous (self-governing) secretors of thyroid hormones. An excessive intake of thyroxine in individuals who are treated for hypothyroidism can cause hyperthyroidism. Rare causes of hyperthyroidism include ectopic thyroid tissue and tumors that secrete TSH although the latter are very uncommon.
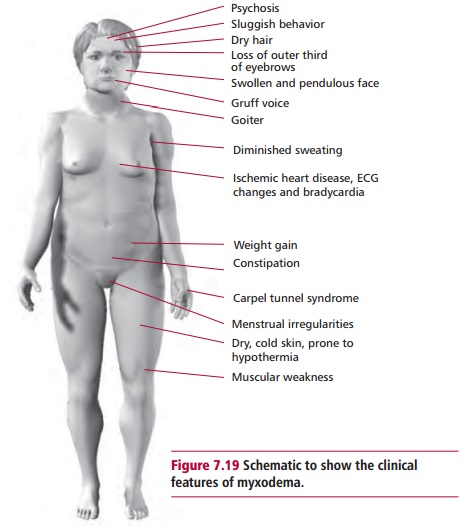
Hypothyroidism (myxodema) is most common in women of 30–60 years of age (Figure 7.19). Its clinical features include psychosis, diminished sweating, hypokinesis, weight gain, muscle weakness, constipation, dry, cold skin and dry hair, ischemic heart disease, bradycardia and menstrual irregularities. The primary cause of hypothyroidism is a defect in the secretion of thyroid hormones by the thyroid gland. In, for example, Hashimoto’s thyroiditis, an autoimmune disease, there is destruction of thyroid tissue by antibodies produced against the thyroid. Hypothyroidism may also occur after surgery (postthyroidectomy) and following treatment with antithyroid drugs. Congenital hypothyroidism occurs because of the failure of the thyroid gland to develop normally during embryonic growth. If untreated, this condition results incretinism where the child suffers from mental retardation, muscle weakness, short stature, neurological signs and is often dumb and mute. Hypothyroidism may also arise due to iodine deficiency in certain parts of the world. Secondary causes of hypothyroidism are linked to the pituitary or the hypothalamus with defective secretions of TSH and TRH respectively.
DIAGNOSIS AND TREATMENT OF THYROID DISORDERS
Investigating thyroid abnormalities involves measurements of serum TSH as a first line test for thyroid function. Some laboratories also include measurement of free T4and/or free or total T 3 in their first line screen. Sensitive assays for measurement of TSH are readily available. Thyroid stimulating hormone levels are increased in primary hypothyroidism, normal in euthyroid individuals (those without thyroid disease) and low or undetectable in hyperthyroidism.
Total T4 and T3 measurements were once used widely in pathology laboratories but have the disadvantage that their values are dependent on plasma TBG levels, which can give misleading results. When TBG levels increase, for example in pregnancy or in females receiving estrogen-containing oral contraceptives, then total T4 and total T3 are also increased even though the individual is not hyperthyroid. Decreases in TBG concentrations occur in malnutrition, protein loss, severe illness and malabsorption and causes a reduction in total T4and T3. There has been some controversyon the validity of free thyroid hormone measurements, but most laboratories now determine free T4and T3, rather than the total concentration. Free T4 and particularly free T3, are usually increased in hyperthyroidism. Free T4 is low in hypothyroidism and is the preferred measurement for its detection because free T3 can be normal in hypothyroidism due to an increase in its peripheral formation from T4. In a few patients with hyperthyroidism, free T4 is within the reference range but free T3 is increased and TSH is nearly always undetectable. This form of hyperthyroidism is referred to as T3toxicosis.
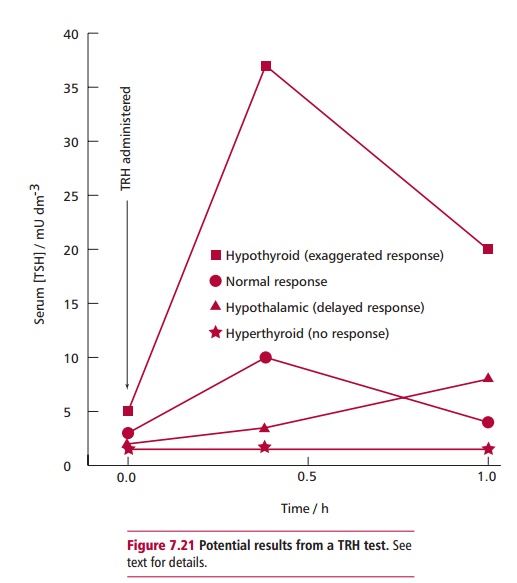
In any systemic illness, such as myocardial infarction, fever or liver disease, the normal metabolism of thyroid hormones is disturbed, reducing the concentrations of T4 and T3 in the plasma because T4 is converted to an inactive isomer called reverse T3 or rT3 (Figure 7.20) and T3 is not replenished from T4. Thyroid stimulating hormone levels may be normal or reduced and concentrations of TBG, albumin and prealbumin may also decline. Patients may have reduced T4, T3 and TSH, although there is no thyroid dysfunction. For this reason, thyroid function tests should not be performed on sick patients until they recover. Table 7.3 outlines the results of tests used in thyroid disorders. The TRH test is rarely used now in the diagnosis of thyroid disease. It is almost exclusively used in the diagnosis of patients with pituitary disease and to assess the capacity of the pituitary to secrete TSH. The patient is given 200 Mg of TRH intravenously and the serum TSH is measured after 0, 20 and 60 min. A normal response involves a three- to fivefold increase in TSH above the basal level. A slow rise in TSH (where the 60-min concentration is greater than the one at 20 min) together with low basal levels of TSH and thyroid hormones suggests hypothalamic disease, while a lack of response is suggestive of pituitary hypothyroidism or hyperthyroidism (Figure 7.21).
Other techniques for investigating thyroid function include administration of isotopes, such as 99mTc-pertechnetate, and determining their distribution using a camera that detects F radiation. This technique distinguishes between active and inactive thyroid nodules and can distinguish between Grave’s disease, multinodular goiter or an adenoma affecting the thyroid gland. A thyroid biopsy involves aspirating tissue from the affected region of the thyroid using a syringe and fine needle. The collected cells are examined microscopically for evidence of thyroid nodules. Often thyroid disease has an autoimmune basis and measurement of antibodies can aid diagnosis. Antiperoxidase and antithyroglobulin antibodies are found in patients affected by Hashimoto’s thyroiditis and often in those with Grave’s disease. However, the detection of antibodies is not always diagnostic, as low levels of these antibodies can occur in older people who are euthyroid.
The management of hyperthyroidism includes using antithyroid drugs, for example, carbimazole. This is useful in young patients and acts by reducing the production of thyroid hormones. Other forms of treatment include radioiodine therapy with 131I, although this is normally used in older patients, and partial or complete surgical removal of the thyroid gland (thyroidectomy). Some patients develop hypothyroidism, often as a consequence of treatment, and may have to be placed on thyroxine therapy. In this regard it is important to monitor TSH levels to detect developing hypothyroidism. Management of hypothyroidism involves replacement therapy with thyroxine, often for life. Thyroxine is readily available, safe and inexpensive. The treatment is monitored at regular intervals by measurement of serum concentrations of TSH to ensure they are kept within its reference range.
Related Topics