Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Quick lime, CaO: Preparation, Properties, Uses
Quick lime, CaO
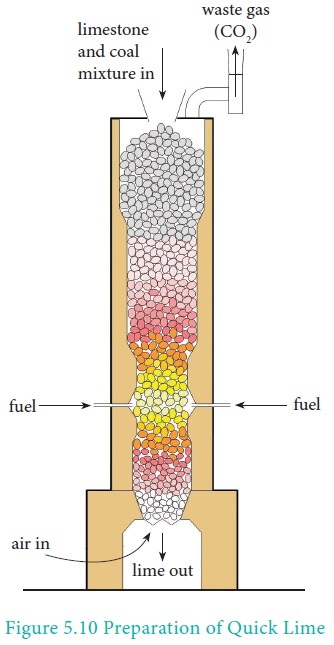
Preparation
It is produced on a commercial scale by heating limestone in a lime kiln in the temperature range 1070-1270K.
CaCO3 ⇌ CaO + CO2
The reaction being reversible, carbon dioxide is removed as soon as it is produced to enable the reaction to proceed to completion.
Properties
Calcium oxide is a white amorphous solid.
It has a melting point of 2870 K.
It absorbs moisture and carbon dioxide on exposure to atmosphere.
CaO + H2O → Ca(OH)2
CaO + CO2 → CaCO3
The addition of limited amount of water breaks the lump of lime. This process is called slaking of lime and the product is slaked lime.
CaO + H2O → Ca(OH)2
Quick lime mixed with soda gives solid soda lime. It combines with acidic oxides such as SiO2 and P4O10 to form CaSiO3 and Ca3(PO4)2, respectively.
CaO + SiO2 → CaSiO3
6CaO + P4O10 → 2Ca3(PO4)2
Uses
Calcium oxide is used
i. to manufacture cement, mortar and glass.
ii. in the manufacture of sodium carbonate and slaked lime.
iii. in the purification of sugar.
iv. as a drying agent.
Related Topics