Preparation, Properties, Uses, Formula | Chemistry - Important compounds of calcium | 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Important compounds of calcium
Important
compounds of calcium
Quick lime, CaO
Preparation
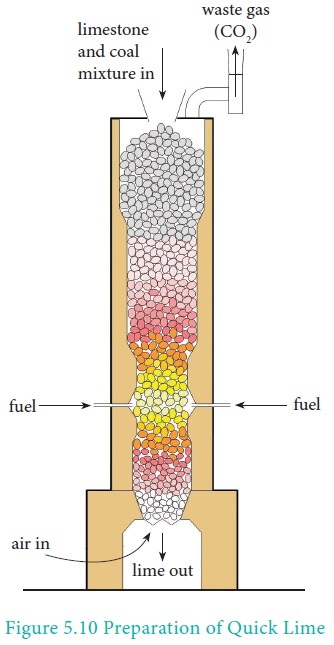
It is produced on a commercial scale by heating limestone
in a lime kiln in the temperature range 1070-1270K.
CaCO3 ⇌ CaO + CO2
The reaction being reversible, carbon dioxide is removed
as soon as it is produced to enable the reaction to proceed to completion.
Properties
Calcium oxide is a white amorphous solid.
It has a melting point of 2870 K.
It absorbs moisture and carbon dioxide on exposure to
atmosphere.
CaO + H2O → Ca(OH)2
CaO + CO2 → CaCO3
The addition of limited amount of water breaks the lump of
lime. This process is called slaking of lime and the product is slaked lime.
CaO + H2O → Ca(OH)2
Quick lime mixed with soda gives solid soda lime. It
combines with acidic oxides such as SiO2 and P4O10
to form CaSiO3 and Ca3(PO4)2,
respectively.
CaO + SiO2 → CaSiO3
6CaO + P4O10 → 2Ca3(PO4)2
Uses
Calcium oxide is used
i.
to manufacture cement, mortar and glass.
ii.
in the manufacture of sodium carbonate and slaked lime.
iii.
in the purification of sugar.
iv.
as a drying agent.
Calcium hydroxide
Preparation
Calcium hydroxide is prepared by adding water to quick
lime, CaO.
Properties
It is a white powder. It is sparingly soluble in water.
The aqueous solution is known as lime water and a suspension of slaked lime in
water is known as milk of lime.
When carbon dioxide is passed through lime water, it turns
milky due to the formation of calcium carbonate.
Ca(OH)2 + CO2 → CaCO3 + H2O
On passing excess of carbon dioxide, the precipitate
dissolves to form calcium hydrogen carbonate.
CaCO3 + CO2 + H2O → Ca(HCO3)2
Milk of lime reacts with chlorine to form hypochlorite, a
constituent of bleaching powder.
2Ca (OH)2 + 2Cl2 → CaCl2 + Ca(OCl)2 + 2H2O
Uses:
Calcium hydroxide is used
i.
in the preparation of mortar, a building material.
ii.
in white wash due to its disinfectant nature.
iii.
in glass making, in tanning industry, in the preparation
of bleaching powder and for the purification of sugar.
Gypsum (CaSO4.2H2O)
Gypsum beds were formed due to the evaporation of water
from the massive prehistoric sea basins. When water evaporates, the minerals
present in it become concentrated, and crystallise.

Properties of Gypsum
·
Gypsum is a soft mineral, which is moderately soluble in
water. The solubility of this mineral in water is affected by temperature.
Unlike other salts, gypsum becomes less soluble in water as the temperature
increases. This is known as retrograde solubility, which is a distinguishing
characteristic of gypsum.
·
Gypsum is usually white, colorless, or gray in color. But
sometimes, it can also be found in the shades of pink, yellow, brown, and light
green, mainly due to the presence of impurities.
·
Gypsum crystals are sometimes found to occur in a form
that resembles the petals of a flower. This type of formation is referred to as
‘desert rose’, as they mostly occur in arid areas or desert terrains.
·
Gypsum is known to have low thermal conductivity, which is
the reason why it is used in making drywalls or wallboards. Gypsum is also
known as a natural insulator.

·
Alabaster is a variety of gypsum, that is highly valued as
an ornamental stone. It has been used by the sculptors for centuries. Alabaster
is granular and opaque.
·
Gypsum has hardness between 1.5 to 2 on Moh’s Hardness
Scale. Its specific gravity is 2.3 to 2.4.
Uses of Gypsum
·
The alabaster variety of gypsum was used in ancient Egypt
and Mesopotamia by the sculptors. The ancient Egyptians knew how to turn gypsum
into plaster of Paris about 5,000 years ago. Today, gypsum has found a wide
range of uses and applications in human society, some of which are enlisted
below.
·
Gypsum is used in making drywalls or plaster boards.
Plaster boards are used as the finish for walls and ceilings, and for
partitions.
·
Another important use of gypsum is the production of
plaster of Paris. Gypsum is heated to about 300 degree Fahrenheit to produce
plaster of Paris, which is also known as gypsum plaster. It is mainly used as a
sculpting material.
·
Gypsum is used in making surgical and orthopedic casts,
such as surgical splints and casting moulds.
·
Gypsum plays an important role in agriculture as a soil
additive, conditioner, and fertilizer. It helps loosen up compact or clay soil,
and provides calcium and sulphur, which are essential for the healthy growth of
a plant. It can also be used for removing sodium from soils having excess
salinity.
·
Gypsum is used in toothpastes, shampoos, and hair
products, mainly due to its binding and thickening properties.
·
Gypsum is a component of Portland cement, where it acts as
a hardening retarder to control the speed at which concrete sets.
·
To sum up, gypsum is one of the most abundant minerals
that have endless uses and applications. Mining of gypsum is simple and easy,
as the mineral occurs in large thick beds near the Earth’s surface. However,
large-scale mining of gypsum involves considerable damage to the environment.
Gypsum can also be recycled, but not much importance has been given to recycle
this mineral due to its abundance.
Plaster
of paris
Calcium Sulphate (Plaster of Paris), CaSO4·½ H2O
It is a hemihydrate of calcium sulphate. It is obtained
when gypsum, CaSO4·2H2O, is heated to 393 K.
2CaSO4 .2H2O(s) →2CaSO4 .H2O+ 3H2O
Above 393 K, no water of crystallisation is left and
anhydrous calcium sulphate, CaSO4 is formed. This is known as ‘dead
burnt plaster’.
It has a remarkable property of setting with water. On
mixing with an adequate quantity of water it forms a plastic mass that gets
into a hard solid in 5 to 15 minutes.
Uses:
The largest use of Plaster of Paris is in the building
industry as well as plasters. It is used for immobilising the affected part of
organ where there is a bone fracture or sprain. It is also employed in
dentistry, in ornamental work and for making casts of statues and busts.

Related Topics