Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
General characteristics of alkali metals
General characteristics of alkali metals:
Alkali metals are highly reactive and are found in nature only as compounds. Rubidium and caesium are found associated in minute quantities with minerals of other alkali metals. Francium is radioactive and does not occur appreciably in nature. Francium is highly radioactive; its longest-lived isotope has a half-life of only 21 minutes.
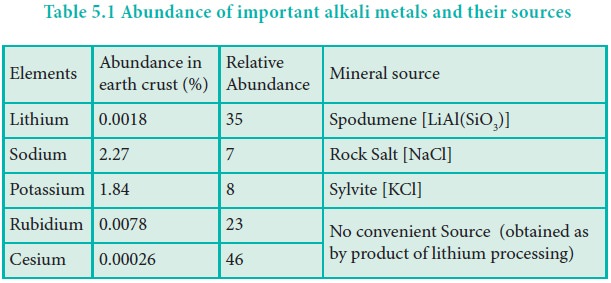

Electronic configuration
The general valence shell electronic configuration of alkali metals is ns1, where ‘n’ represents the period number.
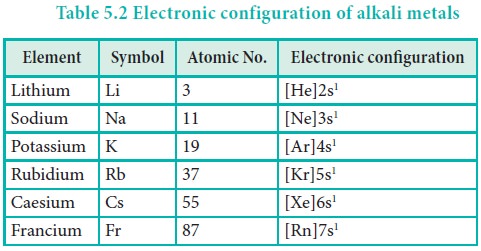
Common oxidation state
All these elements are highly electropositive in nature. They readily lose their valence electron to give monovalent cations (M+). Alkali metals have only one oxidation state which is +1.
Atomic and ionic radii
Being the first element of each period, alkali metals have the largest atomic and ionic radii in their respective periods. On moving down the group, there is an increase in the number of shells and, therefore, atomic and ionic radii increase. The monovalent ions (M+) are smaller than the respective parent atoms as expected.
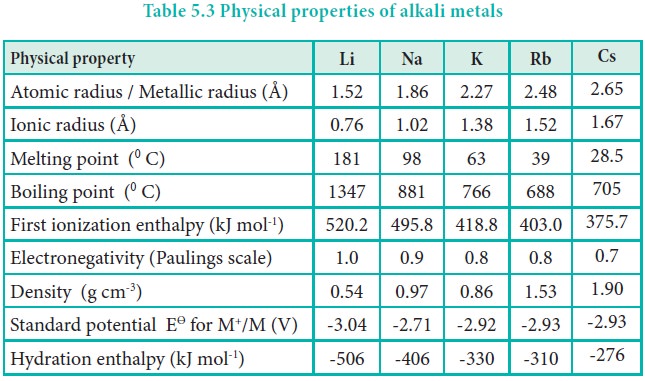
Ionisation enthalpy
Alkali metals have the lowest ionisation enthalpy compared to other elements present in the respective period. As we go down the group, the ionisation enthalpy decreases due to the increase in atomic size. In addition, the number of inner shells also increases, which in turn increases the magnitude of screening effect and consequently, the ionisation enthalpy decreases down the group.
The second ionisation enthalpies of alkali metals are very high. The removal of an electron from the alkali metals gives monovalent cations having stable electronic configurations similar to the noble gas. Therefore, it becomes very difficult to remove the second electron from the stable configurations already attained.
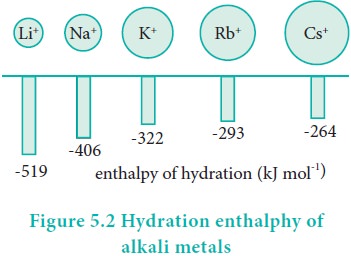
Hydration enthalpy
Lithium salts are more soluble than the salts of other metals of group 1. eg. LiClO4 is up to 12 times more soluble than NaClO4. KClO4, RbClO4 and CsClO4 have solubilities only 10-3 times of that of LiClO4 . The high solubility of Li salts is due to strong solvation of small size of Li+ ion.
Electronegativity:
Alkali metals have comparatively smaller value of electronegativity than the other elements in the respective period. When they react with other elements, they usually produce ionic compounds. For example, they react with halogens to form ionic halides.
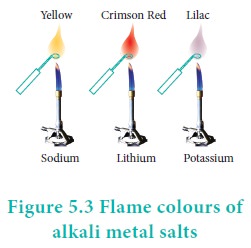
Flame colour and the spectra:
When the alkali metal salts moistened with concentrated hydrochloric acid are heated on a platinum wire in a flame, they show characteristic coloured flame as shown below.
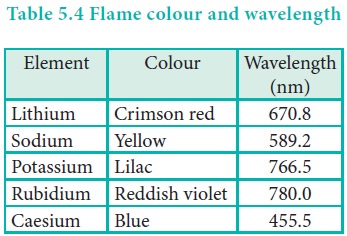
The heat in the flame excites the valence electron to a higher energy level. When it drops back to its actual energy level, the excess energy is emitted as light, whose wavelength is in the visible region as shown in the above table.
Related Topics