General characteristics, Distinctive behavior of beryllium, Chemical properties, Uses - Alkaline earth metals | 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Alkaline earth metals
Alkaline
earth metals
Group 2 in the modern periodic table contains the elements
beryllium, magnesium, calcium, strontium, barium and radium. These elements
with the exception of beryllium are commonly known as the alkaline earth metals
because their oxides and hydroxides are alkaline in nature and these metal
oxides are found in the earth’s crust.

General characteristics of alkaline earth metals
Physical state
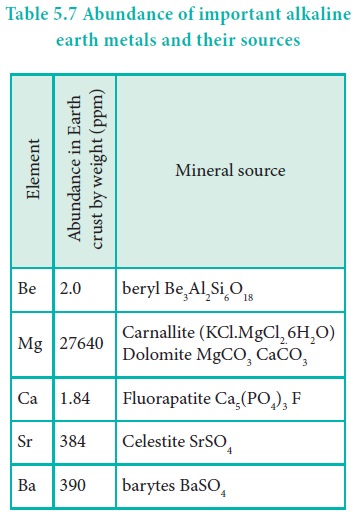
Beryllium is rare and radium is the rarest of all
comprising only 10 % of igneous rocks. Magnesium and calcium are very common in
the earth’s crust, with calcium the fifth-most-abundant element, and magnesium
the eighth. Magnesium and calcium are found in many rocks and minerals:
magnesium in carnallite, magnesite, dolomite and calcium in chalk, limestone,
gypsum. Most strontium is found in the minerals celestite and strontianite.
Barium is slightly less common, much of it in the mineral barite. Radium, being
a decay product of uranium, is found in all uranium-bearing ores.
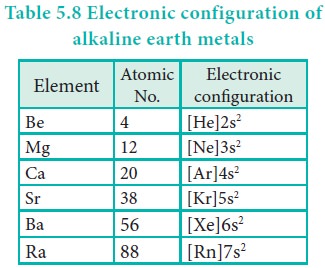
Electronic configuration
These elements have two electrons in the valence shell of
their atoms, preceded by the noble gas configuration. Their general electronic
configuration is written as [Noble gas]ns2 where ‘n’ represents the
valence shell.
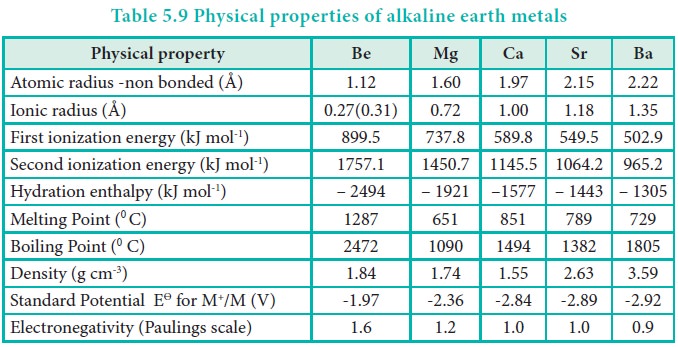
Atomic and ionic radii
The atomic and ionic radii of alkaline earth metals are
smaller than the corresponding members of the alkali metals. This is due to the
fact the Group 2 elements having a higher nuclear charge that allows electrons
to be attracted more strongly towards the nucleus. On moving down the group,
the radii increases due to gradual increase in the number of the shells and the
screening effect.
Common oxidation state
The group 2 elements have two electrons in their valence
shell and by losing these electrons, they acquire the stable noble gas
configuration. So these elements exhibit +2 oxidation state in their compounds.
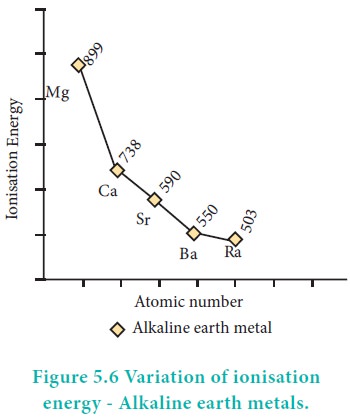
Ionisation enthalpy
Due to a fairly large size of the atoms, alkaline earth
metals have low ionisation enthalpies when compared to ‘p’ block elements. Down
the group the ionisation enthalpy decreases as atomic size increases. This is
due to the addition of new shells as well as increase in the magnitude of the
screening effect of inner shell electrons. Members of group 2 have higher
ionization enthalpy values than group 1 because of their smaller size, with
electrons being more attracted towards the nucleus of the atoms.
Correspondingly they are less electropositive than alkali metals.
Although IE1 values of alkaline earth metals
are higher than that of alkali metals, the IE2 values of alkaline
earth metals are much smaller than those of alkali metals. This occurs because
in alkali metals the second electron is to be removed from a cation, which has
already acquired a noble gas configuration. In the case of alkaline earth
metals, the second electron is to be removed from a monovalent cation, which
still has one electron in the outermost shell. Thus, the second electron can be
removed more easily in the case of group 2 elements than in group 1 elements.
Hydration Enthalpies
Compounds of alkaline earth metals are more extensively
hydrated than those of alkali metals, because the hydration enthalpies of
alkaline earth metal ions are larger than those of alkali metal ions.
Like alkali metal ions, the hydration enthalpies of
alkaline earth metal ions also decrease with increase in ionic size down the
group.
Be > Mg > Ca > Sr > Ba
e.g., MgCl2 and CaCl2 exist as MgCl2.6H2O
and CaCl2· 6H2O while NaCl and KCl do not form such
hydrates.
Electronegativity
In alkaline earth metals the electronegativity values
decrease as we go down the group as seen in the alkali metals.
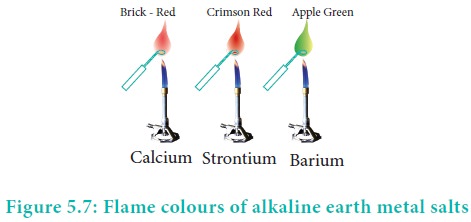
Flame colour and the spectra:
When the alkaline earth metal salts moistened with
concentrated hydrochloric acid are heated on a platinum wire in a flame, they
show characteristic coloured flame as shown below.
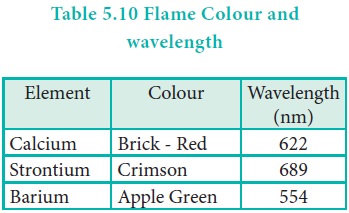
The heat in the flame excites the valence electron to a
higher energy level. when it drops back to its actual energy level, the excess
energy is emitted as light, whose wavelength is in the visible region as shown
in the above table.
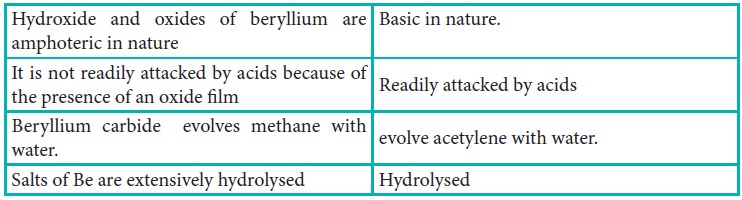
Distinctive behavior of beryllium
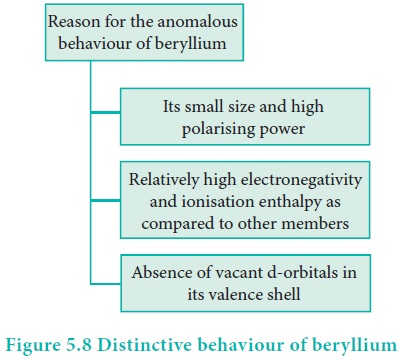
The anomalous properties of beryllium is mainly due to its
small size, high electronegativity, high ionisation energy and high polarising
power compared to the other elements in the block. The anomalous properties of
beryllium compared to other elements of the group are mentioned in Table 5.11
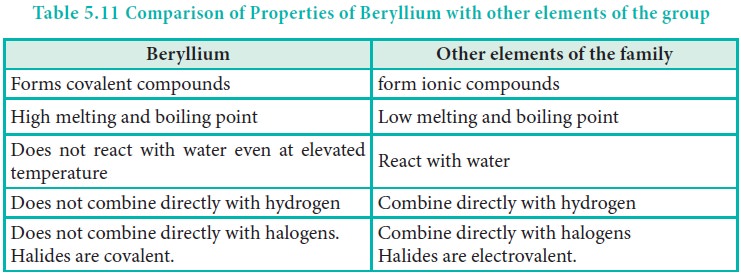
Diagonal Relationship:
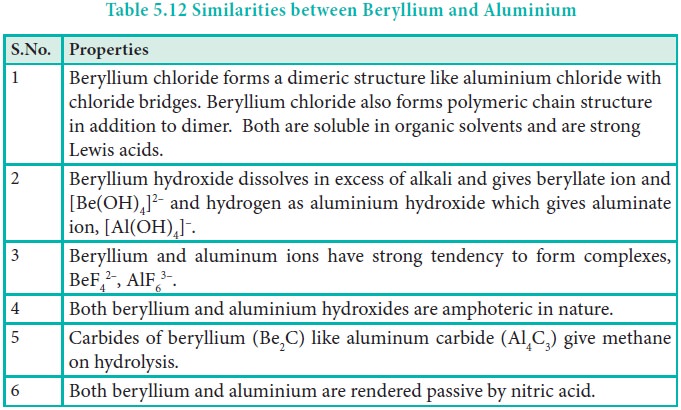
As observed in alkali metals, beryllium (the first member
of group 2) shows a diagonal relationship with aluminium. In this case, the
size of these ions (rBe2+ = 0.45 Å and rAl3+ = 0.54 Å) is
not as close. However, their charge per unit area is closer (Be2+ =
2.36 and Al3+ = 2.50). They also have same electronegativity values
(Be = 1.5; Al = 1.5).
Chemical properties of alkaline earth metals
The alkaline earth metals are less reactive than the
alkali metals. The reactivity of these elements increases on going down the
group.
Reactivity towards the halogens:
All the alkaline earth metals combine with halogen at
elevated temperatures to form their halides.
M + X2 → MX2
(M= Be, Mg, Ca, Sr, Ba, Ra , X = F, Cl, Br, l )
Thermal decomposition of (NH4)2BeF4
is the best route for the preparation of BeF2. BeCl2 is
conveniently made from the oxide.
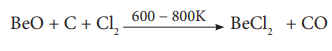
Reactivity towards hydrogen:
All the elements except beryllium, combine with hydrogen
on heating to form their hydrides with general formula MH2. BeH2
can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Uses of alkaline earth metals
Uses of beryllium
1. Because of its low atomic number and very low
absorption for X-rays, it is used as radiation windows for X-ray tubes and
X-ray detectors.
2. The sample holder in X-ray emission studies usually
made of beryllium
3. Since beryllium is transparent to energetic particles
it is used to build the ‘beam pipe’ in accelerators.
4. Because of its low density and diamagnetic nature, it
is used in various detectors.
Uses of magnesium
1. Removal of sulphur from iron and steel
2. Refining of titanium
in the “Kroll” process.
3. Used as photoengrave plates in printing industry.
4. Magnesium alloys are used in aeroplane and missile
construction.
5. Mg ribbon is used in synthesis of Grignard reagent in
organic synthesis.
6. It alloys with aluminium to improve its mechanical,
fabrication and welding property.
7. As a desiccant .
8. As sacrificial anode in controlling galvanic corrosion.
Uses of calcium
1. As a reducing agent in the metallurgy of uranium,
zirconium and thorium.
2. As a deoxidiser, desulphuriser or decarboniser for
various ferrous and non-ferrous alloys.
3. In making cement and mortar to be used in construction.
4. As a getter in vacuum tubes.
5. In dehydrating oils
6. In fertilisers, concrete and plaster of paris.
Uses of strontium
1. 90Sr is used in cancer therapy.
2. 87Sr / 86Sr ratios are
commonly used in marine investigations as well as in teeth, tracking animal
migrations or in criminal forensics.
3. Dating of rocks.
4. As a radioactive tracer in determining the source of
ancient archaeological materials such as timbers and coins.
Uses of Barium
1. Used in metallurgy, its compounds are used in
pyrotechnics, petroleum mining and radiology.
2. Deoxidiser in copper refining.
3. Its alloys with nickel readily emits electrons hence
used in electron tubes and in spark plug electrodes.
4. As a scavenger to remove last traces of oxygen and
other gases in television and other electronic tubes.
5. An isotope of barium 133Ba, used as a source
in the calibration of gamma ray detectors in nuclear chemistry.
Uses of Radium
Used in self-luminous paints for watches, nuclear panels,
aircraft switches, clocks and instrument dials.
Related Topics