Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chemical properties of alkali metals
Chemical properties of alkali metals
Alkali metals exhibit high chemical reactivity. The reactivity of alkali metals increases from Li to Cs, since the ionisation energy decreases down the group. All alkali metals are highly reactive towards the more electronegative elements such as oxygen and halogens. Some characteristic chemical properties of alkali metals are described blow.
Reaction with oxygen
All the alkali metals on exposure to air or oxygen burn vigorously, forming oxides on their surface. Lithium forms only monoxide, sodium forms the monoxide and peroxide and the other elements form monoxide, peroxide, and superoxides. These oxides are basic in nature.
4Li +O2 → 2Li2O (simple oxide)
2Na +O2 → Na2O2 (peroxide)
M + O2 → MO2
(M= K, Rb,Cs; MO2 -superoxide)
Reaction with hydrogen
All alkali metals react with hydrogen at about 673 K (lithium at 1073 K) to form the corresponding ionic hydrides. Reactivity of alkali metals with hydrogen decreases from Li to Cs.
2M + H2 →2 M+H-
(M = Li, Na, K, Rb, Cs)
The ionic character of the hydrides increases from Li to Cs and their stability decreases. The hydrides behave as strong reducing agents and their reducing nature increases down the group.
Reaction with halogen
Alkali metals combine readily with halogens to form ionic halides MX. Reactivity of alkali metals with halogens increases down the group because of corresponding decrease in ionisation enthalpy.
2M + X2 → 2 MX
(M= Li, Na, K, Rb, Cs) (X= F, Cl, Br, I)
All metal halides are ionic crystals. However Lithium iodide shows covalent character, as it is the smallest cation that exerts high polarising power on the iodide anion. Additionally, the iodide ion being the largest can be polarised to a greater extent by Li+ ion.
Reaction with liquid ammonia:
Alkali metals dissolve in liquid ammonia to give deep blue solutions that are conducting in nature. The conductivity is similar to that of pure metals (The specific conductivity of Hg is 104 Ω-1 and for sodium in liquid ammonia is 0.5 x 104 Ω-1). This happens because the alkali metal atom readily loses its valence electron in ammonia solution. Both the cation and the electron are ammoniated to give ammoniated cation and ammoniated electron.
M + (x + y)NH3 → [M(NH3)x ]+ + [e(NH3)y ]−
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of an amide.
M+ + e −+ NH3 MNH2+ ½H2
In concentrated solution, the blue colour changes to bronze colour and become diamagnetic.
Reaction with water:
Alkali metals react with water to give corresponding hydroxides with the liberation of hydrogen.
2 Li + 2 H2O → 2 LiOH+ H2
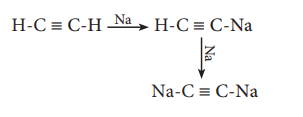
They also react with alcohol, and alkynes which contain active hydrogens.
Na + 2 C2H5OH → 2 C2H5ONa + H2
Reducing activity:
Alkali metals can lose their valence electron readily hence they act as good reducing agents.
M(s) → M+(g) + e–
Reaction with carbon:
Lithium directly reacts with carbon to form the ionic compound, lithium carbide. Other metals do not react with carbon directly. However, when they are treated with compounds like acetylene they form acetelydes.
2 Li + 2C → Li2C2
Related Topics