Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Distinctive behavior of lithium
The distinctive behaviour of Li+ ion is due to its exceptionally small size, high polarising power, high hydration energy and non availability of d-orbitals.
Distinctive behavior of lithium
The distinctive behaviour of Li+ ion is due to its exceptionally small size, high polarising power, high hydration energy and non availability of d-orbitals.
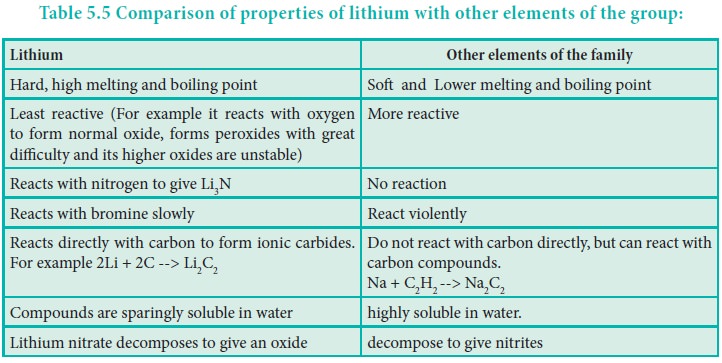
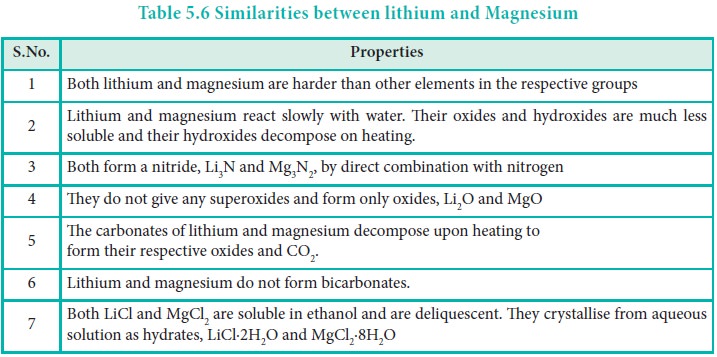
Diagonal Relationship:
Similarity between the first member of group 1 (Li) and the diagonally placed second element of group 2 (Mg) is called diagonal relationship. It is due to similar size (r Li+ = 0.766 Å and Mg2+ = 0.72 Å) and comparable electronegativity values (Li = 1.0; Mg = 1.2).
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals : Distinctive behavior of lithium |
Related Topics
11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals