with Answers and Solution - Choose the best Answer: Alkali and Alkaline Earth Metals (Chemistry) | 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Choose the best Answer: Alkali and Alkaline Earth Metals (Chemistry)
Alkali and Alkaline Earth Metals | Chemistry
Choose the best Answer
1. For alkali metals, which one of the following trends is incorrect ?
a) Hydration energy : Li > Na > K > Rb
b) Ionisation energy : Li > Na > K > Rb
c) Density : Li < Na < K < Rb
d) Atomic size : Li < Na < K < Rb
Answer: c) Density : Li < Na < K < Rb
Solution:
Potassium is lighter than sodium (Refer table 5.3)
The correct order of density is Li < K < Na < Rb < Cs
0.54 < 0.86 < 0.97< 1.53< 1.90(in g cm–3)
2. Which of the following statements is incorrect ?
a) Li+ has minimum degree of hydration among alkali metal cations.
b) The oxidation state of K in KO2 is +1
c) Sodium is used to make Na / Pb alloy
d) MgSO4 is readily soluble in water
Answer: a) Li+ has minimum degree of hydration among alkali metal cations.
Solution:
Li+ has maximum degree of hydration among alkali metal cations.
Li+ > Na+ > K+ > Rb+ > Cs+
3. Which of the following compounds will not evolve H2 gas on reaction with alkali metals ?
a) ethanoic acid
b) ethanol
c) phenol
d) none of these
Answer: d) none of these
Solution:
All these compounds reacts with alkali metals to evolve hydrogen gas.
4. Which of the following has the highest tendency to give the reaction M+(g) --Aqueous---Medium → M+(aq)
a) Na
b) Li
c) Rb
d) K
Answer: b) Li
Solution:
hydration energy of Li+ is more and hence Li+ is stabilized in aqueous medium.
5. Sodium is stored in
a) alcohol
b) water
c) kerosene
d) none of these
Answer: c) kerosene
6. RbO2 is
a) superoxide and paramagnetic
b) peroxide and diamagnetic
c) superoxide and diamagnetic
d) peroxide and paramagnetic
Answer: a) superoxide and paramagnetic
Solution:
RbO2 is a super oxide which contains Rb+ and O2– ions. O2– contains one unpaired electron and hence it is paramagnetic.
7. Find the wrong statement
a) sodium metal is used in organic qualitative analysis
b) sodium carbonate is soluble in water and it is used in inorganic qualitative analysis
c) potassium carbonate can be prepared by solvay process
d) potassium bicarbonate is acidic salt
Answer: c) potassium carbonate can be prepared by solvay process
Solution:
Potassium carbonate cannot be prepared by solvay process. Potassium bicarbonate is fairly soluble in water and does not precipitate out.
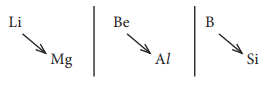
8. Lithium shows diagonal relationship with
a) sodium
b) magnesium
c) calcium
d) aluminium
Answer: b) magnesium
Solution:
9. Incase of alkali metal halides, the ionic character increases in the order
a) MF < MCl < MBr < MI
b) MI < MBr < MCl < MF
c) MI < MBr < MF < MCl
d) none of these
Answer: b) MI < MBr < MCl < MF
Solution:
ionic character (difference in electronegativity)
MI < MBr < MCl < MF
10. In which process, fused sodium hydroxide is electrolysed for extraction of sodium ?
a) Castner's process
b) Cyanide process
c) Down process
d) All of these
Answer: a) Castner's process
Solution:
Castner's process
NaOH ↔ Na+ + OH–
Cathode : Na+ + e– → Na
Anode : 2OH– → H2O + 1/2O2 + 2e–
11. The product obtained as a result of a reaction of nitrogen with CaC2 is
a) Ca(CN)3
b) CaN2
c) Ca(CN)2
d) Ca3N2
Answer: c) Ca(CN)2
Solution:
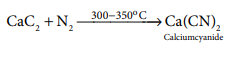
12. Which of the following has highest hydration energy
a) MgCl2
b) CaCl2
c) BaCl2
d) SrCl2
Answer: a) MgCl2
Solution:
MgCl2
The order of hydration energy of alkaline earth metal is
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
13. Match the flame colours of the alkali and alkaline earth metal salts in the bunsen burner
(p) Sodium (1) Brick red
(q) Calcium (2) Yellow
(r) Barium (3) Violet
(s) Strontium (4) Apple green
(t) Cesium (5) Crimson red
(u) Potassium (6) Blue
a) p - 2, q - 1, r - 4, s - 5, t - 6, u - 3
b) p - 1, q - 2, r - 4, s - 5, t - 6, u - 3
c) p - 4, q - 1, r - 2, s - 3, t - 5, u - 6
d) p - 6, q - 5, r - 4, s - 3, t - 1, u - 2
Answer: a) p - 2, q - 1, r - 4, s - 5, t - 6, u - 3
Solution:
(p) sodium –yellow (2)
(q) Calcium – Brick red (1)
(r) Barium – apple green (4)
(s) Strontium – Crimson red (5)
(t) Cesium – blue (6)
(u) Potassium – Violet (3)
14. Assertion : Generally alkali and alkaline earth metals form superoxides
Reason : There is a single bond between O and O in superoxides.
a) both assertion and reason are true and reason is the correct explanation of asser-tion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false
Answer: d) both assertion and reason are false
Solution:
Among alkali and alkaline earth metals,
K, Rb and Cs alone forms superoxides.
Superoxide O2– has 3 electron bond.
15. Assertion : BeSO4 is soluble in water while BaSO4 is not
Reason : Hydration energy decreases down the group from Be to Ba and lattice energy remains almost constant.
a) both assertion and reason are true and reason is the correct explanation of asser-tion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false
Answer: a) both assertion and reason are true and reason is the correct explanation of asser-tion
Solution:
both are true and reason is the correct explanation of assertion.
16. Which is the correct sequence of solubility of carbonates of alkaline earth metals ?
a) BaCO3 > SrCO3 > CaCO3 > MgCO3
b) MgCO3 > CaCO3 > SrCO3 > BaCO3
c) CaCO3 > BaCO3 > SrCO3 > MgCO3
d) BaCO3 > CaCO3 > SrCO3 > MgCO3
Answer: b) MgCO3 > CaCO3 > SrCO3 > BaCO3
Solution:
Solubility of carbonates decreases down the group and hence the correct order of solubility is,
MgCO3 > CaCO3 > SrCO3 > BaCO3
17. In context with beryllium, which one of the following statements is incorrect ?
a) It is rendered passive by nitric acid
b) It forms Be2C
c) Its salts are rarely hydrolysed
d) Its hydride is electron deficient and polymeric
Answer: c) Its salts are rarely hydrolysed
Solution:
Correct Statement: Beryllium salts are easily hydrolysed
18. The suspension of slaked lime in water is known as
a) lime water
b) quick lime
c) milk of lime
d) aqueous solution of slaked lime
Answer: c) milk of lime
Solution:
Slaked lime Ca(OH)2
The suspension is called milk of lime and the clear solution is called lime water.
19. A colourless solid substance (A) on heating evolved CO2 and also gave a white resi-due, soluble in water. Residue also gave CO2 when treated with dilute HCl.
a) Na2CO3
b) NaHCO3
c) CaCO3
d) Ca(HCO3)2
Answer: b) NaHCO3
Solution:
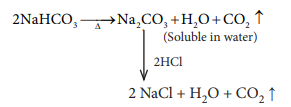
20. The compound (X) on heating gives a colourless gas and a residue that is dissolved in water to obtain (B). Excess of CO2 is bubbled through aqueous solution of B, C is formed. Solid (C) on heating gives back X. (B) is
a) CaCO3
b) Ca(OH)2
c) Na2CO3
d) NaHCO3
Answer: b) Ca(OH)2
Solution:
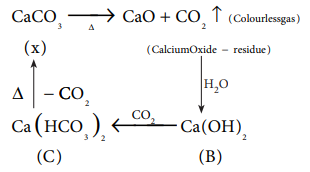
21. Which of the following statement is false ?
a) Ca2+ ions are not important in maintaining the regular beating of the heart
b) Mg2+ ions are important in the green parts of the plants
c) Mg2+ ions form a complex with ATP
d) Ca2+ ions are important in blood clotting
Answer: a) Ca2+ ions are not important in maintaining the regular beating of the heart
Solution:
Ca2+ ion plays an important role in maintaining regular heart beat.
22. The name 'Blue John' is given to which of the following compounds ?
a) CaH2
b) CaF2
c) Ca3(PO4)2
d) CaO
Answer: b) CaF2
Solution:
'Blue john' – CaF2 (A variety of fluorite)
23. Formula of Gypsum is
a) CaSO4 . 2H2O
b) CaSO4 . ½ H2O
c) 3CaSO4 . H2O
d) 2CaSO4 . 2H2O
Answer: a) CaSO4 . 2H2O
Solution:
CaSO4 . 2H2O∴ Option (a)
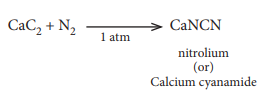
24. When CaC2 is heated in atmospheric nitrogen in an electric furnace the compound formed is
a) Ca(CN)2
b) CaNCN
c) CaC2N2
d) CaNC2
Answer: b) CaNCN
Solution:
25. Among the following the least thermally stable is
(a) K2CO3
b) Na2CO3
(c) BaCo3
d) Li2CO3
Answer: d) Li2CO3
Solution:
Li2CO3 is least stable.
Related Topics