Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Distinctive behavior of beryllium
Distinctive behavior of beryllium
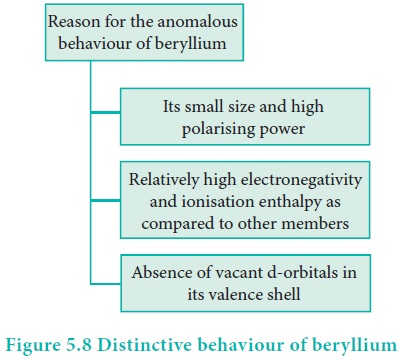
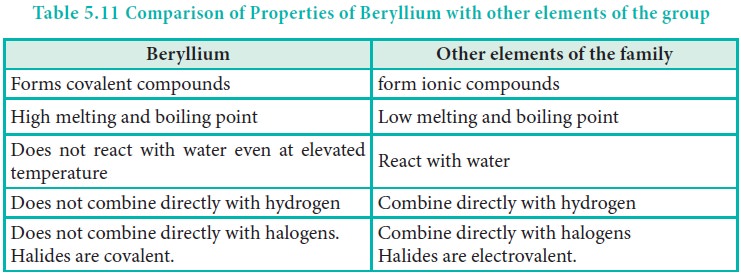
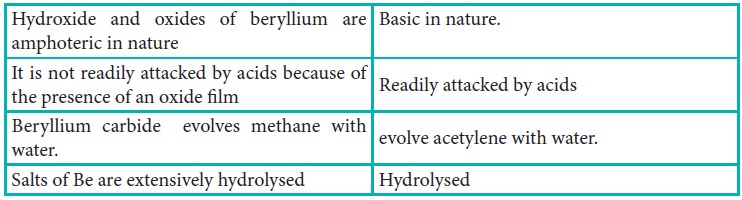
The anomalous properties of beryllium is mainly due to its small size, high electronegativity, high ionisation energy and high polarising power compared to the other elements in the block. The anomalous properties of beryllium compared to other elements of the group are mentioned in Table 5.11
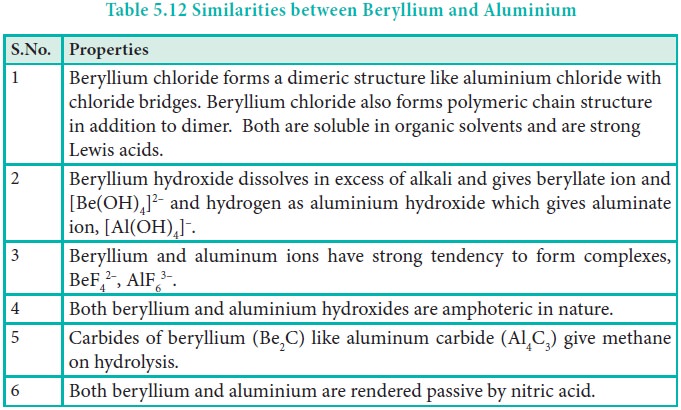
Diagonal Relationship:
As observed in alkali metals, beryllium (the first member of group 2) shows a diagonal relationship with aluminium. In this case, the size of these ions (rBe2+ = 0.45 Å and rAl3+ = 0.54 Å) is not as close. However, their charge per unit area is closer (Be2+ = 2.36 and Al3+ = 2.50). They also have same electronegativity values (Be = 1.5; Al = 1.5).
Related Topics