General characteristics, Electronic configuration, Electronegativity, Distinctive behavior, Chemical properties, Uses - Alkali metals | 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Alkali metals
Alkali metals:
The word “alkali” is derived from the word al-qalīy
meaning the plant ashes, referring to the original source of alkaline
substances. A water-extract of burnt plant ashes, called potash contain mainly
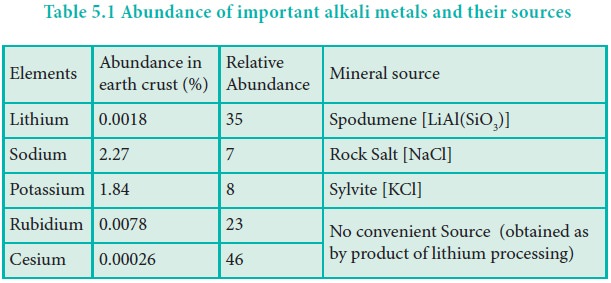
potassium carbonate. Alkali metal group consists of the elements: lithium,
sodium, potassium, rubidium, caesium and francium. They are all metals,
generally soft and highly reactive. They form oxides and hydroxides and these
compounds are basic in nature.
General characteristics of alkali metals:
Alkali metals are highly reactive and are found in nature
only as compounds. Rubidium and caesium are found associated in minute
quantities with minerals of other alkali metals. Francium is radioactive and
does not occur appreciably in nature. Francium is highly radioactive; its
longest-lived isotope has a half-life of only 21 minutes.

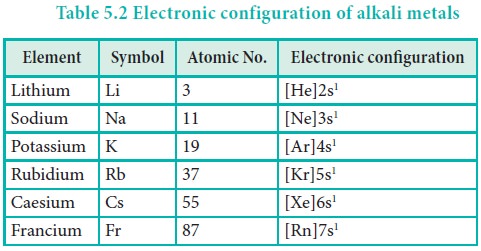
Electronic configuration
The general valence shell electronic configuration of
alkali metals is ns1, where ‘n’ represents the period number.
Common oxidation state
All these elements are highly electropositive in nature.
They readily lose their valence electron to give monovalent cations (M+).
Alkali metals have only one oxidation state which is +1.
Atomic and ionic radii
Being the first element of each period, alkali metals have
the largest atomic and ionic radii in their respective periods. On moving down
the group, there is an increase in the number of shells and, therefore, atomic
and ionic radii increase. The monovalent ions (M+) are smaller than
the respective parent atoms as expected.
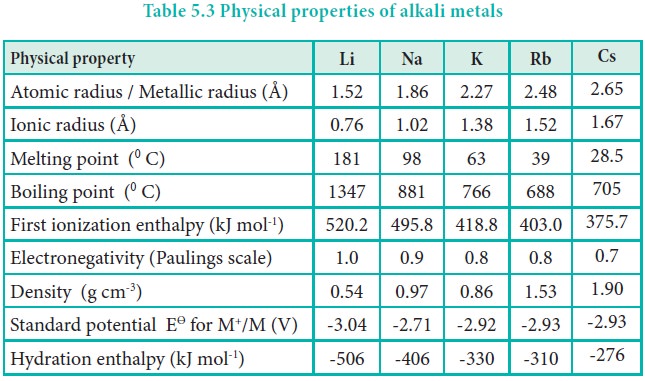
Ionisation enthalpy
Alkali metals have the lowest ionisation enthalpy compared
to other elements present in the respective period. As we go down the group,
the ionisation enthalpy decreases due to the increase in atomic size. In
addition, the number of inner shells also increases, which in turn increases
the magnitude of screening effect and consequently, the ionisation enthalpy
decreases down the group.
The second ionisation enthalpies of alkali metals are very
high. The removal of an electron from the alkali metals gives monovalent
cations having stable electronic configurations similar to the noble gas.
Therefore, it becomes very difficult to remove the second electron from the
stable configurations already attained.
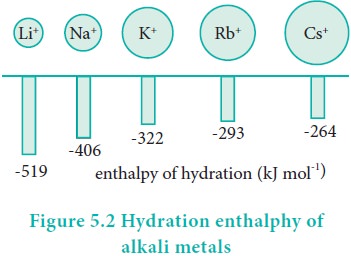
Hydration enthalpy
Lithium salts are more soluble than the salts of other
metals of group 1. eg. LiClO4 is up to 12 times more soluble than
NaClO4. KClO4, RbClO4 and CsClO4
have solubilities only 10-3 times of that of LiClO4 . The
high solubility of Li salts is due to strong solvation of small size of Li+
ion.
Electronegativity:
Alkali metals have comparatively smaller value of
electronegativity than the other elements in the respective period. When they
react with other elements, they usually produce ionic compounds. For example,
they react with halogens to form ionic halides.
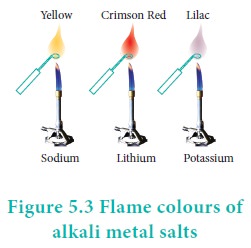
Flame colour and the spectra:
When the alkali metal salts moistened with concentrated
hydrochloric acid are heated on a platinum wire in a flame, they show
characteristic coloured flame as shown below.
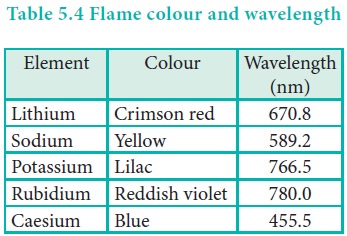
The heat in the flame excites the valence electron to a
higher energy level. When it drops back to its actual energy level, the excess
energy is emitted as light, whose wavelength is in the visible region as shown
in the above table.
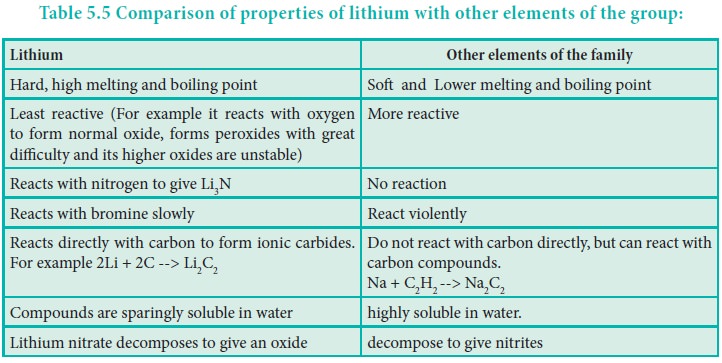
Distinctive behavior of lithium
The distinctive behaviour of Li+ ion is due to
its exceptionally small size, high polarising power, high hydration energy and
non availability of d-orbitals.
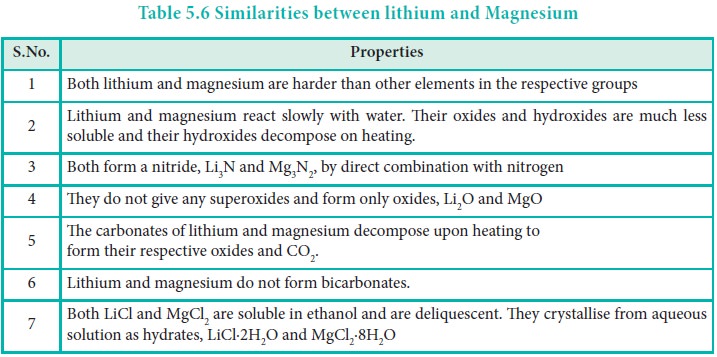
Diagonal Relationship:
Similarity between the first member of group 1 (Li) and
the diagonally placed second element of group 2 (Mg) is called diagonal
relationship. It is due to similar size (r Li+ = 0.766 Å and Mg2+
= 0.72 Å) and comparable electronegativity values (Li = 1.0; Mg = 1.2).
Chemical
properties of
alkali metals
Alkali metals exhibit high chemical reactivity. The
reactivity of alkali metals increases from Li to Cs, since the ionisation
energy decreases down the group. All alkali metals are highly reactive towards
the more electronegative elements such as oxygen and halogens. Some
characteristic chemical properties of alkali metals are described blow.
Reaction with oxygen
All the alkali metals on exposure to air or oxygen burn
vigorously, forming oxides on their surface. Lithium forms only monoxide, sodium
forms the monoxide and peroxide and the other elements form monoxide, peroxide,
and superoxides. These oxides are basic in nature.
4Li +O2 →
2Li2O (simple oxide)
2Na +O2 → Na2O2 (peroxide)
M + O2 →
MO2
(M= K, Rb,Cs; MO2 -superoxide)
Reaction with hydrogen
All alkali metals react with hydrogen at about 673 K
(lithium at 1073 K) to form the corresponding ionic hydrides. Reactivity of alkali
metals with hydrogen decreases from Li to Cs.
2M + H2 →2 M+H-
(M = Li, Na, K, Rb, Cs)
The ionic character of the hydrides increases from Li to Cs and their
stability decreases. The hydrides behave as strong reducing agents and their
reducing nature increases down the group.
Reaction with halogen
Alkali metals combine readily with halogens to form ionic
halides MX. Reactivity of alkali metals with halogens increases down the group
because of corresponding decrease in ionisation enthalpy.
2M + X2 →
2 MX
(M= Li, Na, K, Rb, Cs) (X= F, Cl, Br, I)
All metal halides are ionic crystals. However Lithium
iodide shows covalent character, as it is the smallest cation that exerts high
polarising power on the iodide anion. Additionally, the iodide ion being the largest
can be polarised to a greater extent by Li+ ion.
Reaction with liquid ammonia:
Alkali metals dissolve in liquid ammonia to give deep blue
solutions that are conducting in nature. The conductivity is similar to that of
pure metals (The specific conductivity of Hg is 104 Ω-1
and for sodium in liquid ammonia is 0.5 x 104 Ω-1). This
happens because the alkali metal atom readily loses its valence electron in
ammonia solution. Both the cation and the electron are ammoniated to give
ammoniated cation and ammoniated electron.
M + (x + y)NH3 → [M(NH3)x
]+ + [e(NH3)y ]−
The blue colour of the solution is due to the ammoniated
electron which absorbs energy in the visible region of light and thus imparts
blue colour to the solution. The solutions are paramagnetic and on standing
slowly liberate hydrogen resulting in the formation of an amide.
M+ + e −+ NH3 MNH2+ ½H2
In concentrated solution, the blue colour changes to
bronze colour and become diamagnetic.
Reaction with water:
Alkali metals react with water to give corresponding
hydroxides with the liberation of hydrogen.
2 Li + 2 H2O → 2 LiOH+ H2
They also react with alcohol, and alkynes which contain
active hydrogens.
Na + 2 C2H5OH → 2 C2H5ONa + H2
Reducing activity:
Alkali metals can lose their valence electron readily
hence they act as good reducing agents.
M(s) → M+(g) + e–
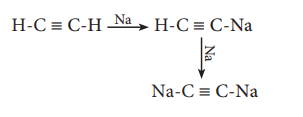
Reaction with carbon:
Lithium directly reacts with carbon to form the ionic
compound, lithium carbide. Other metals do not react with carbon directly. However,
when they are treated with compounds like acetylene they form acetelydes.
2 Li + 2C → Li2C2
Uses of alkali metals:
i. Lithium metal is used to make useful alloys. For
example with lead it is used to make ‘white metal’ bearings for motor engines,
with aluminium to make aircraft parts, and with magnesium to make armour
plates. It is used in thermonuclear reactions.
ii. Lithium is also used to make electrochemical cells.
iii. Lithium carbonate is used in medicines
iv. Sodium is used to make Na/Pb alloy needed to make
Pb(Et)4 and Pb(Me)4. These organolead compounds were
earlier used as anti-knock additives to petrol, but nowadays lead-free petrol
in use.
v. Liquid sodium metal is used as a coolant in fast
breeder nuclear reactors. Potassium has vital role in biological systems.
vi. Potassium chloride is used as a fertilizer. Potassium
hydroxide is used in the manufacture of soft soap. It is also used as an
excellent absorbent of carbon dioxide.
vii. Caesium is used in devising photoelectric cells.
Related Topics