Chapter: 11th Chemistry : UNIT 5 : Alkali and Alkaline Earth Metals
Chemical properties of alkaline earth metals
Chemical properties of alkaline earth metals
The alkaline earth metals are less reactive than the alkali metals. The reactivity of these elements increases on going down the group.
Reactivity towards the halogens:
All the alkaline earth metals combine with halogen at elevated temperatures to form their halides.
M + X2 → MX2
(M= Be, Mg, Ca, Sr, Ba, Ra , X = F, Cl, Br, l )
Thermal decomposition of (NH4)2BeF4 is the best route for the preparation of BeF2. BeCl2 is conveniently made from the oxide.
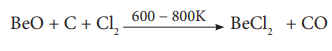
Reactivity towards hydrogen:
All the elements except beryllium, combine with hydrogen on heating to form their hydrides with general formula MH2. BeH2 can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Related Topics