Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Properties of Hydrogen
Properties
of Hydrogen
Physical Properties:
Hydrogen is a colorless, odorless, tasteless, lightest and
highly flammable gas. It is a non-polar diatomic molecule. It can be liquefied
under low temperature and high pressure. Hydrogen is a good reducing agent.
Various physical constants of hydrogen molecule are listed in Table 4.1.
Chemical Properties:
Hydrogen reacts with oxygen to give water. This is an
explosive reaction and releases lot of energy. This is used in fuel cells to
generate electricity.
2 H2 + O2
→ 2 H2O
Similarly, hydrogen also reacts with halogens to give
corresponding halides. Reaction with fluorine takes place even in dark with
explosive violence while with chlorine at room temperature under light. It
combines with bromine on heating and reaction with iodine is a photochemical
reaction.
H2 + X2 → 2 HX (X = F, Cl, Br &
I)
In the above reactions the hydrogen has an oxidation state
of +1. It also has a tendency to react with reactive metals such as lithium,
sodium and calcium to give corresponding hydrides in which the oxidation state
of hydrogen is -1.
2 Li + H2 → 2 LiH
2 Na + H2 → 2 NaH
These hydrides are used as reducing agents in synthetic
organic chemistry. It is used to prepare other important hydrides such as
lithium aluminium hydride and sodium boro hydride.
4 LiH + AlCl3 → Li[AlH4] + 3 LiCl
4 NaH + B(OCH3)3 → Na[BH4]
+ 3 CH3ONa
Hydrogen itself acts as a reducing agent. In the presence
of finely divided nickel, it adds to the unsaturated organic compounds to form
saturated compounds.
HC=CH - -- Ni / H 2 → H 2 C = CH 2 - - Ni /H2 → H 3 C - CH3
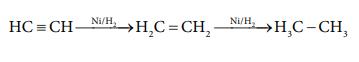
Chemical properties of Deuterium
Like hydrogen, deuterium also reacts with oxygen to form
deuterium oxide called heavy water. It also reacts with halogen to give
corresponding halides.
2 D2 + O2 → 2 D2O
D2 + X2 → 2 DX
(X = F, Cl, Br & I)
Deuterium exchange reactions:
Deuterium can replace reversibly hydrogen in compounds
either partially or completely depending upon the reaction conditions. These
reactions occur in the presence of deuterium or heavy water.
CH4 + 2 D2 → CD4 + 2 H2
2 NH3 + 3 D2 → 2 ND3 + 3
H2
Properties of Tritium
It is a β-emitter with a half-life period of 12.3 years .
13 T → 32 He +10
e
Related Topics