Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Ortho- and Para-Hydrogen
Ortho− and Para−Hydrogen:
In the hydrogen atom, the nucleus has a spin. When molecular hydrogen is formed, the spins of two hydrogen nuclei can be in the same direction or in the opposite direction as shown in the figure. These two forms of hydrogen molecules are called ortho and para hydrogens respectively.
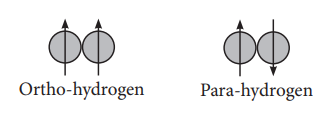
At room temperature, normal hydrogen consists of about 75% ortho-form and 25% para-form. As the ortho-form is more stable than para-form, the conversion of one isomer into the other is a slow process. However, the equilibrium shifts in favour of para hydrogen when the temperature is lowered. The para-form can be catalytically transformed into ortho-form using platinum or iron. Alternatively, it can also be converted by passing an electric discharge, heating above 800°C and mixing with paramagnetic molecules such as O2, NO, NO2 or with nascent/atomic hydrogen.
Ortho and para hydrogen are similar in chemical properties but differ in some of the physical properties. For example, the melting point of para hydrogen is 13.83 K while that of ortho-H2 13.95 K; boiling point of para hydrogen is 20.26 K while that of ortho hydrogen 20.39 K. Since the nuclear spins are in opposite directions the magnetic moment of para hydrogen is zero and ortho hydrogen has magnetic moment twice that of a proton.
Related Topics