with Answers and Solution - Choose the best Answer: Hydrogen (Chemistry) | 11th Chemistry : UNIT 4 : Hydrogen
Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Choose the best Answer: Hydrogen (Chemistry)
Hydrogen (Chemistry)
Choose the best Answer
1. Which of the following statements about hydrogen is incorrect ?
a) Hydrogen ion, H3O+ exists freely in solution.
b) Dihydrogen acts as a reducing agent.
c) Hydrogen has three isotopes of which tritium is the most common.
d) Hydrogen never acts as cation in ionic salts.
Answer: c) Hydrogen has three isotopes of which tritium is the most common.
Solution:
Correct statement : Hydrogen has three isotopes of which protium is the most common.
2. Water gas is
a) H2O (g)
b) CO + H2O
c) CO + H2
d) CO + N2
Answer: c) CO + H2
Solution:
CO + H2 - Water gas
3. Which one of the following statements is incorrect with regard to ortho and para dihydrogen ?
a) They are nuclear spin isomers
b) Ortho isomer has zero nuclear spin whereas the para isomer has one nuclear spin
c) The para isomer is favoured at low temperatures
d) The thermal conductivity of the para isomer is 50% greater than that of the ortho isomer.
Answer: b) Ortho isomer has zero nuclear spin whereas the para isomer has one nuclear spin
Solution:
Correct statement : Ortho isomer - one nuclear spin
Para isomer - zero nuclear spin
4. Ionic hydrides are formed by
a) halogens
b) chalogens
c) inert gases
d) group one elements
Answer: d) group one elements
Solution:
eg : Sodium hydride (Na+ H–)
5. Tritium nucleus contains
a) 1p + 0 n
b) 2 p + 1n
c) 1p + 2n
d) none of these
Answer: c) 1p + 2n
Solution:
1T3 (1e–, 1p, 2n)
6. Non-stoichiometric hydrides are formed by
a) palladium, vanadium
b) carbon, nickel
c) manganese, lithium
d) nitrogen, chlorine
Answer: a) palladium, vanadium
7. Assertion : Permanent hardness of water is removed by treatment with washing soda.
Reason : Washing soda reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates
a) Both assertion and reason are true and reason is the correct explanation of assertion.
b) Both assertion and reason are true but reason is not the correct explanation of assertion.
c) Assertion is true but reason is false
d) Both assertion and reason are false
Answer: a) Both assertion and reason are true and reason is the correct explanation of assertion.
Solution:
Ca2+ + Na2CO3 → CaCO3 ↓ + 2Na+
8. If a body of a fish contains 1.2 g hydrogen in its total body mass, if all the hydrogen is replaced with deuterium then the increase in body weight of the fish will be
a) 1.2 g
b) 2.4 g
c) 3.6 g
d) √4.8 g
Answer: a) 1.2 g
Solution:
mass of deuterium = 2 × mass of protium
If all the 1.2 g hydrogen is replaced with deuterium, the weight will become 2.4g. Hence the increase in body weight is (2.4 – 1.2 = 1.2 g)
9. The hardness of water can be determined by volumetrically using the reagent
a) sodium thio sulphate
b) potassium permanganate
c) hydrogen peroxide
d) EDTA
Answer: d) EDTA
10. The cause of permanent hardness of water is due to
a) Ca(HCO3)2
b) Mg(HCO3)2
c) CaCl2
d) MgCO3
Answer: c) CaCl2
Solution:
Permanent hardness if water is due to the presence of the chlorides, nitrates and sulphates of Ca2+ and Mg2+ ions.
11. Zeolite used to soften hardness of water is, hydrated
a) Sodium aluminium silicate
b) Calcium aluminium silicate
c) Zinc aluminium borate
d) Lithium aluminium hydride
Answer: a) Sodium aluminium silicate
Solution:
Zeolite is sodium aluminium silicate. (NaAlSi2O6 . H2O)
12. A commercial sample of hydrogen peroxide marked as 100 volume H2O2, it means that
a) 1 ml of H2O2 will give 100 ml O2 at STP
b) 1 L of H2O2 will give 100 ml O2 at STP
c) 1 L of H2O2 will give 22.4 L O2
d) 1 ml of H2O2 will give 1 mole of O2 at STP
Answer: a) 1 ml of H2O2 will give 100 ml O2 at STP
Solution:
(a) 1 mL of H2O2 will give 100ml O2 at STP.
13. When hydrogen peroxide is shaken with an acidified solution of potassium dichromate in presence of ether, the ethereal layer turns blue due to the formation of
a) Cr2O3
b) CrO42–
c) CrO(O2)2
d) none of these
Answer: c) CrO(O2)2
Solution:
Cr2O72– + 2H+ + 4H2O2 → 2CrO(O2)2 + 5H2O
14. For decolourisation of 1 mole of acidified KMnO4, the moles of H2O2 required is
a) 1/2
b) 3/2
c) 5/2
d) 7/2
Answer: c) 5/2
Solution:
2MnO4– + 5H2O2(aq) + 6H+ → 2Mn2+ + 5O2 + 8H2O
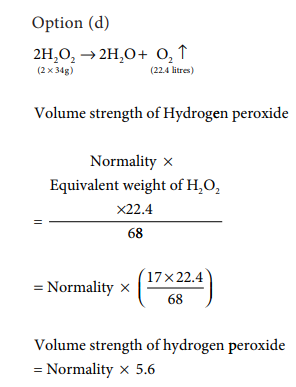
15. Volume strength of 1.5 N H2O2 is
a) 1.5
b) 4.5
c) 16.8
d) 8.4
Answer: d) 8.4
Volume strength of hydrogen peroxide = Normality of hydrogen peroxide × 5.6
=1.5 × 5.6
=8.4
16. The hybridisation of oxygen atom is H2O and H2O2 are, respectively
a) sp and sp3
b) sp and sp
c) sp and sp2
d) sp3 and sp3
Answer: d) sp3 and sp3
17. The reaction H3PO2 + D2O → H2DPO2 + HDO indicates that hypo-phosphorus acid is
a) tribasic acid
b) dibasic acid
c) mono basic acid
d) none of these
Answer: c) mono basic acid
Solution:
Hypophosphorus acid on reaction with D2O, only one hydrogen is replaced by deuterium and hence it is mono basic
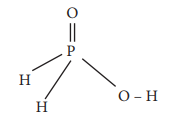
Option (c) monobasic acid
18. In solid ice, oxygen atom is surrounded
a) tetrahedrally by 4 hydrogen atoms
b) octahedrally by 2 oxygen and 4 hydrogen atoms
c) tetrahedrally by 2 hydrogen and 2 oxygen atoms
d) octahedrally by 6 hydrogen atoms
Answer: a) tetrahedrally by 4 hydrogen atoms
Solution:
(a) tetrahedrally surrounded by 4 hydrogen atoms (refer 4.6 (a) Structure of ice)
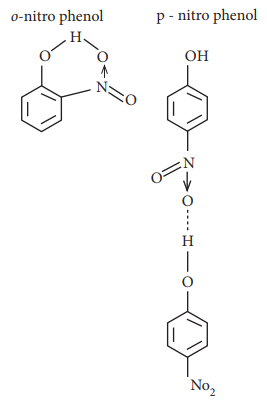
19. The type of H-bonding present in ortho nitro phenol and p-nitro phenol are respectively
a) inter molecular H-bonding and intra molecular H-bonding
b) intra molecular H-bonding and inter molecular H-bonding
c) intra molecular H - bonding and no H - bonding
d) intra molecular H - bonding and intra molecular H – bonding
Answer: b) intra molecular H-bonding and inter molecular H-bonding
Solution:
20. Heavy water is used as
a) modulator in nuclear reactions
b) coolant in nuclear reactions
c) both (a) and (b)
d) none of these
Answer: c) both (a) and (b)
Solution:
Heavy water is used as moderator as well as coolant in nuclear reactions.
21. Water is a
a) basic oxide
b) acidic oxide
c) amphoteric oxide
d) none of these
Answer: c) amphoteric oxide
Solution:
Water is a amphoteric oxide.
Related Topics