Definition, Formula, properties, Uses - Heavy Water | 11th Chemistry : UNIT 4 : Hydrogen
Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Heavy Water
Heavy
Water:
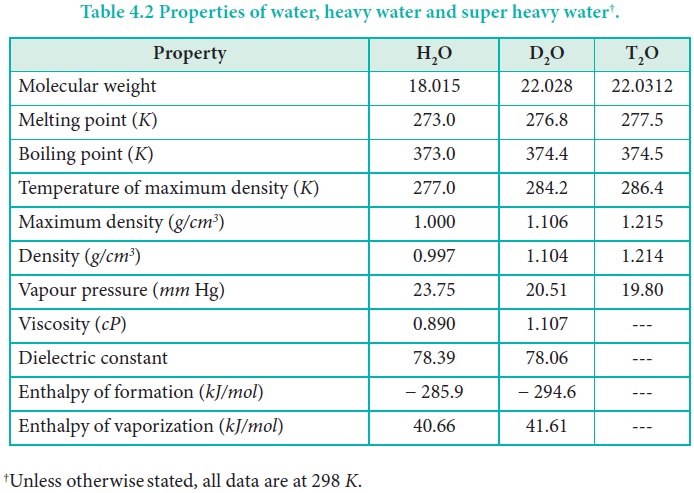
Heavy water (D2O) is the oxide of heavy hydrogen.
One part of heavy water is present in 5000 parts of ordinary water. It is
mainly obtained as the product of electrolysis of water, as D2O does
not undergo electrolysis as easily as H2O.
D2O is a colorless, odorless and tasteless
liquid. However, there is a marked difference between physical properties of
water and heavy water as shown in Table 4.2.
Hard water
produces less foam with detergents. Do you know why?
The cleaning capacity of soap is reduced when used in hard
water. Soaps are sodium or potassium salts of long chain fatty acids (e.g.,
coconut oil). When soap is added to hard water, the divalent magnesium and
calcium ions present in hard water react with soap. The sodium salts present in
soaps are converted to their corresponding magnesium and calcium salts which
are precipitated as scum/precipitate.
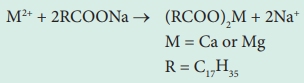
Chemical properties of heavy water:
When compounds containing hydrogen are treated with D2O,
hydrogen undergoes an exchange for deuterium
2NaOH + D2O → 2NaOD + HOD
HCl + D2O → DCl + HOD
NH4Cl + 4D2O → ND4Cl +
4HOD
These exchange reactions are useful in determining the
number of ionic hydrogens present in a given compound.
For example, when D2O is treated with of
hypo-phosphorus acid only one hydrogen atom is exchanged with deuterium. It
indicates that, it is a monobasic acid.
H3PO2 + D2O → H2DPO2
+ HDO
It is also used to prepare some deuterium compounds:
Al4C3 + 12D2O → 4Al(OD)3
+ 3CD4
CaC2 + 2 D2O → Ca(OD)2 +
C2D2
Mg3N2 + 6D2O → 3Mg(OD)2
+ 2 ND3
Ca3P2 + 6D2O → 3Ca(OD)2
+ 2PD3
Uses of heavy water:
1. Heavy water is widely used as moderator in nuclear
reactors as it can lower the energies of fast neutrons
2. It is commonly used as a tracer to study organic
reaction mechanisms and mechanism of metabolic reactions
3. It is also used as a coolant in nuclear reactors as it
absorbs the heat generated.
Related Topics