Definition, Formula, Structure, properties, Uses - Hydrogen Peroxide | 11th Chemistry : UNIT 4 : Hydrogen
Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Hydrogen Peroxide
Hydrogen
Peroxide:
Hydrogen peroxide (H2O2) is one of
the most important peroxides. It can be prepared by treating metal peroxide
with dilute acid.
BaO2 + H2SO4 → BaSO4
+ H2O2
Na2O2 + H2SO4
→ Na2SO4 + H2O2
On an industrial scale, hydrogen peroxide is now prepared
exclusively by autoxidation of 2-alkyl anthraquinol.
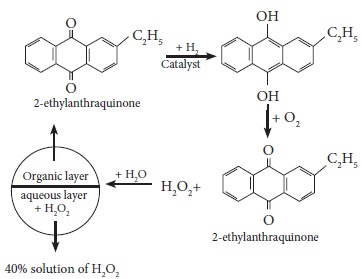
Physical properties:
Pure hydrogen peroxide is almost a colorless liquid (pale
blue), less volatile and more viscous than water.
A 30 % solution of hydrogen peroxide is marketed as
‘100-volume’ hydrogen peroxide indicating that at S.T.P., 100 ml of oxygen is
liberated by 1 ml of this solution on heating.
Chemical properties:
Hydrogen peroxide is highly unstable and the aqueous
solution spontaneously disproportionates to give oxygen and water. The reaction
is, however, slow but is explosive when catalyzed by metal. If it is stored in
glass container, it dissolves the alkali metals from the glass, which catalyzes
the disproportionation reaction. For this reason, H2O2
solutions are stored in plastic bottles.
H2O2 → H2O + ½O2
Hydrogen peroxide can act both as an oxidizing agent and a
reducing agent. Oxidation is usually performed in acidic medium while the
reduction reactions are performed in basic medium
In acidic conditions:
H2O2 + 2 H+ +2 e-
→ 2 H2O (E0 = + 1.77 V)
For example
2FeSO4+H2SO4 + H2O2
→ Fe2(SO4)3 + 2H2O
In basic conditions:
HO2- + OH- oO2
+ H2O + 2 e-
(E0 = + 0.08 V)
For Example,
2KMnO4 (aq) + 3 H2O2 (aq)
↓
2MnO2 + 2 KOH + 2 H2O +3O2
(g)
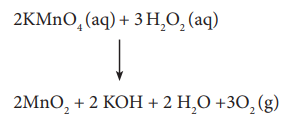
Uses of hydrogen peroxide:
The oxidizing ability of hydrogen peroxide and the
harmless nature of its products, i.e., water and oxygen, lead to its many
applications. It is used in water treatment to oxidize pollutants, as a mild
antiseptic, and as bleach in textile, paper and hair-care industry.
Hydrogen peroxide is used to restore the white colour of
the old paintings which was lost due to the reaction of hydrogen sulphide in
air with the white pigment Pb3(OH)2(CO3)2
to form black colored lead sulphide. Hydrogen peroxide oxidises black coloured
lead sulphide to white coloured lead sulphate, there by restoring the colour.
PbS + 4H2O2 → PbSO4 + 4 H2O
Structure of hydrogen peroxide:
Both in gas-phase and liquid-phase, the molecule adopts a
skew conformation due to repulsive interaction of the OH bonds with lone-pairs
of electrons on each oxygen atom. Indeed, it is the smallest molecule known to
show hindered rotation about a single bond.
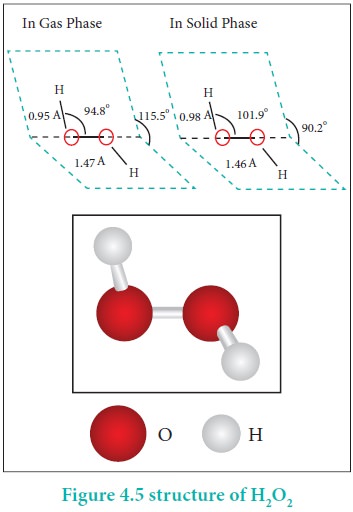
H2O2 has a non-polar structure. The
molecular dimensions in the gas phase and solid phase differ as shown in figure
4.5. Structurally, H2O2 is represented by the dihydroxyl
formula in which the two OH-groups do not lie in the same plane. One
way of explaining the shape of hydrogen peroxide is that the hydrogen atoms
would lie on the pages of a partly opened book, and the oxygen atoms along the
spine. In the solid phase of molecule, the dihedral angle reduces to 90.2o
due to hydrogen bonding and the O-O-H angle expands from
94.8 º to 101.9o.
Related Topics