Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Physical and Chemical Properties of Water
Physical Properties:
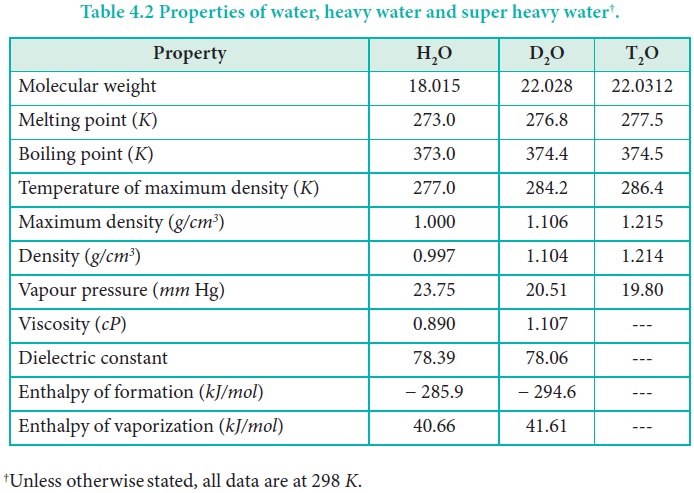
Water is a colourless and volatile liquid. The peculiar properties of water in the condensed phases are due to the presence of inter molecular hydrogen bonding between water molecules. Hydrogen bonding is responsible for the high melting and boiling points of water. Some of the physical parameters of water are listed in Table 4.2.
Chemical Properties:
Water reacts with metals, non-metals and other compounds differently. The most reactive metals are the alkali metals. They decompose water even in cold with the evolution of hydrogen leaving an alkali solution.
2Na + 2 H2O → 2 NaOH + H2
The group 2 metals (except beryllium) react in a similar way but less violently. The hydroxides are less soluble than those of Group 1.
Ba + 2H2O → Ba(OH)2 + H2
Some transition metals react with hot water or steam to form the corresponding oxides. For example, steam passed over red hot iron results in the formation of iron oxide with the release of hydrogen.
3Fe + 4H2O → Fe3O4 + H2
Lead and copper decompose water only at a white heat. Silver, gold, mercury and platinum do not have any effect on water. In the elemental form, the non-metals such as carbon, sulphur and phosphorus normally do not react with water. However, as we have seen earlier, carbon will react with steam when it is red (or white) hot to give water gas.
On the other hand, the halogens react with water to give an acidic solution. For example, chlorine forms hydrochloric acid and hypo chlorous acid. It is responsible for the antibacterial action of chlorine water, and for its use as bleach.
Cl2 + H2O → HCl + HOCl
Fluorine reacts differently to liberate oxygen from water.
2F2 + 2 H2O → 4HF + O2
In a similar way, compounds of non-metals react with water to give acidic or alkaline solutions. For example, solutions of carbonates are slightly alkaline.
CO32− + H2O → HCO3− + OH−
Water is an amphoteric oxide. It has the ability to accept as well as donate protons and hence it can act as an acid or a base. For example, in the reaction with HCl it accepts proton where as in the reaction with weak base ammonia it donates proton.
NH3 + H2O → NH4+ + OH−
HCl + H2O → H3O+ + Cl−
Water dissolves ionic compounds. In addition, it also hydrolyses some covalent compounds.
SiCl4 + 2 H2O → SiO2 + 4 HCl
P4O10 + 6 H2O → 4 H3PO4
Many salts crystallized from aqueous solutions form hydrated crystals. The water in the hydrated salts may form co-ordinate bond or just present in interstitial positions of crystals.
Examples: [Cr(H2O)6]Cl3 – All six water molecules form co-ordinate bond
BaCl2.2H2O – Both the water molecules are present in interstitial positions.
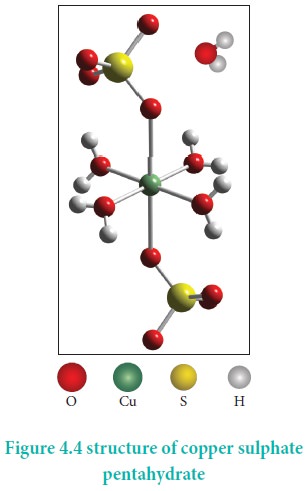
CuSO4.5H2O – In this compound four water molecules form co-ordinate bonds while the fifth water molecule, present outside the co-ordination, can form intermolecular hydrogen bond with another molecule. [Cu(H2O)4]SO4.H2O
Related Topics