Laboratory, Industrial Preparation - Preparation of Hydrogen | 11th Chemistry : UNIT 4 : Hydrogen
Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Preparation of Hydrogen
Preparation
of Hydrogen
High purity hydrogen (>99.9 %) is obtained by the
electrolysis of water containing traces of acid or alkali or the electrolysis
of aqueous solution of sodium hydroxide or potassium hydroxide using a nickel
anode and iron cathode. However, this process is not economical for large-scale
production.
At anode : 2 OH- → H2O + ½ O2
+ 2e-
At cathode : 2 H2O + 2 e- → 2 OH-
+ H2
Overall reaction : H2O → H2 + ½ O2
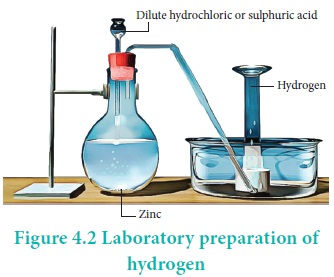
Laboratory Preparation
Hydrogen is conveniently prepared in laboratory by the
reaction of metals, such as zinc, iron, tin with dilute acid.
Zn + 2 HCl → ZnCl2 + H2↑
Industrial Production
In the large-scale, hydrogen is produced by
steam-reforming of hydrocarbons. In this method hydrocarbon such as methane is
mixed with steam and passed over nickel catalyst in the range 800-900 °C and 35 atm pressures.
CH4 + H2O → CO + 3H2
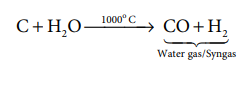
In an another process, steam is passed over a red-hot coke
to produce carbon monoxide and hydrogen. The mixture of gases produced in this
way is known as water gas (CO+H2).
This is also called syngas (Synthetic
gas) as it is used in the synthesis of organic compounds such as methanol and
simple hydrocarbons.
Conversion of Carbon monoxide in water gas to Carbon dioxide:
The carbon monoxide of the water gas can be converted to
carbon dioxide by mixing the gas mixture with more steam at 400°C and passed over a shift
converter containing iron/copper catalyst. This reaction is called as water-gas shift reaction.
CO + H2O → CO2 + H2
The CO2 formed in the above process is absorbed
in a solution of potassium carbonate.
CO2 + K2CO3 + H2O
→ 2 KHCO3
Preparation of Deuterium:
Electrolysis of heavy water:
Normal water contains 1.6 x 10-4 percentage of
heavy water. The dissociation of protium water (H2O) is more than
heavy water (D2O). Therefore, when water is electrolysed, hydrogen
is liberated much faster than D2. The electrolysis is continued
until the resulting solution becomes enriched in heavy water. Further
electrolysis of the heavy water gives deuterium.
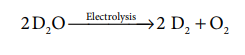
2D2 O -- Electrolysis
→2 D2 + O2
Preparation of Tritium:
As explained earlier the tritium is present only in trace
amounts. So it can be artificially prepared by bombarding lithium with slow
neutrons in a nuclear fission reactor. The nuclear transmutation reaction for
this process is as follows.
63 Li + 10 n → 24
He +13 T
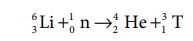
Related Topics