Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Isotopes of Hydrogen
Isotopes of Hydrogen
Hydrogen has three naturally occurring isotopes, viz., protium (1H1 or H), deuterium (1H2 or D) and tritium (1H3 or T). Protium (1H1) is the predominant form (99.985 %) and it is the only isotope that does not contain a neutron.
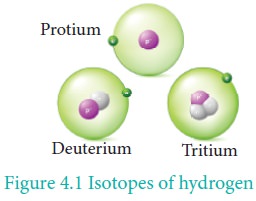
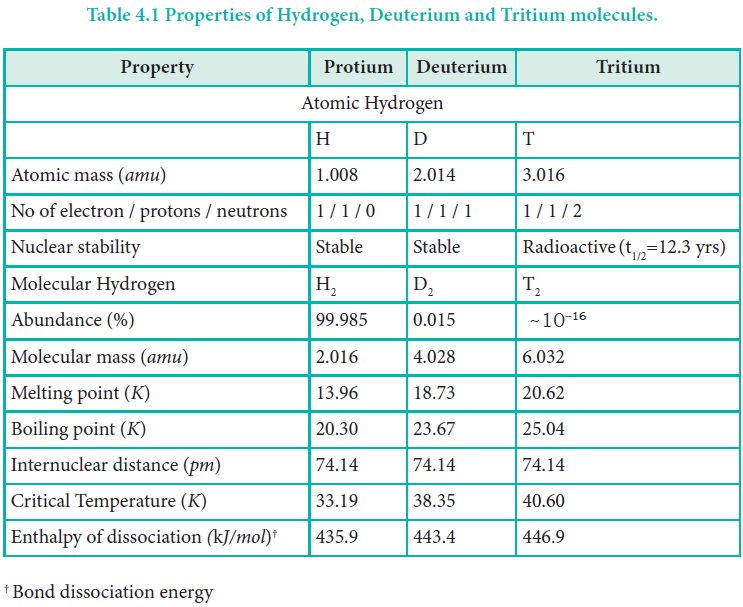
Deuterium, also known as heavy hydrogen, constitutes about 0.015 %. The third isotope, tritium is a radioactive isotope of hydrogen which occurs only in traces (~1 atom per 1018 hydrogen atoms). Due to the existence of these isotopes naturally occurring hydrogen exists as H2, HD, D2, HT, T2, and DT. The properties of these isotopes are shown in Table 4.1.
Table 4.1 Properties of Hydrogen, Deuterium and Tritium molecules.
Related Topics