Chapter: Basic & Clinical Pharmacology : Antiprotozoal Drugs
Primaquine - Malaria
PRIMAQUINE
Primaquine is the drug
of choice for the eradication of dormant liver forms of P vivax and P ovale and
can also be used for chemo-prophylaxis against all malarial species.
Chemistry & Pharmacokinetics
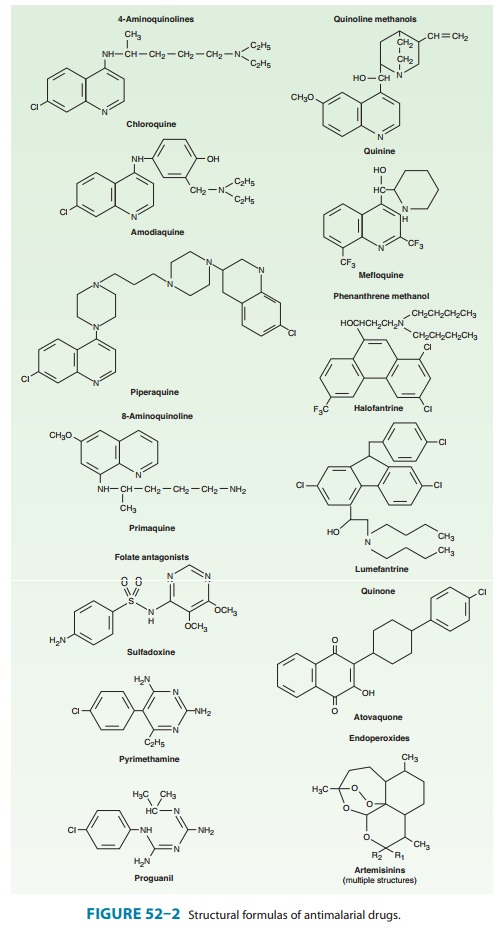
Primaquine phosphate
is a synthetic 8-aminoquinoline (Figure 52–2). The drug is well absorbed
orally, reaching peak plasma levels in 1–2 hours. The plasma half-life is 3–8
hours. Primaquine is widely distributed to the tissues, but only a small amount
is bound there. It is rapidly metabolized and excreted in the urine. Its three
major metabolites appear to have less antimalarial activity but more potential
for inducing hemolysis than the parent compound.
Antimalarial Action & Resistance
Primaquine
is active against hepatic stages of all human malaria parasites. It is the only
available agent active against the dormant hypnozoite stages of P vivax and P ovale. Primaquine is also gameto-cidal against the four human
malaria species. Primaquine acts against erythrocytic stage parasites, but this
activity is too weak to play an important role. The mechanism of antimalarial
action is unknown.
Some
strains of P vivax in New Guinea,
Southeast Asia, Central and South America, and other areas are relatively
resistant to primaquine. Liver forms of these strains may not be eradicated by
a single standard treatment with primaquine and may require repeated therapy.
Because of decreasing efficacy, the standard dos-age of primaquine for radical
cure of P vivax infection was
recently doubled to 30 mg base daily for 14 days.
Clinical Uses
A. Therapy (Radical Cure) of Acute Vivax and Ovale Malaria
Standard therapy for
these infections includes chloroquine to eradi-cate erythrocytic forms and
primaquine to eradicate liver hypnozo-ites and prevent a subsequent relapse.
Chloroquine is given acutely, and therapy with primaquine is withheld until the
G6PD status of the patient is known. If the G6PD level is normal, a 14-day
course of primaquine is given. Prompt evaluation of the G6PD level is helpful,
since primaquine appears to be most effective when insti-tuted before
completion of dosing with chloroquine.
B. Terminal Prophylaxis of Vivax and Ovale Malaria
Standard
chemoprophylaxis does not prevent a relapse of vivax or ovale malaria, because
the hypnozoite forms of these parasites are not eradicated by chloroquine or
other available blood schizonti-cide agents. To markedly diminish the
likelihood of relapse, some authorities advocate the use of primaquine after
the completion of travel to an endemic area.
C. Chemoprophylaxis of Malaria
Primaquine has been
studied as a daily chemoprophylactic agent. Daily treatment with 30 mg (0.5
mg/kg) of base provided good levels of protection against falciparum and vivax
malaria. However, potential toxicities of long-term use remain a concern, and
pri-maquine is generally recommended for this purpose only when mefloquine,
Malarone, and doxycycline cannot be used.
D. Gametocidal Action
A single dose of
primaquine (45 mg base) can be used as a control measure to render P falciparum gametocytes noninfective to
mos-quitoes. This therapy is of no clinical benefit to the patient but will
disrupt transmission.
E. Pneumocystis
jiroveci Infection
The combination of
clindamycin and primaquine is an alternative regimen in the treatment of
pneumocystosis, particularly mild to moderate disease. This regimen offers
improved tolerance com-pared with high-dose trimethoprim-sulfamethoxazole or
pentami-dine, although its efficacy against severe pneumocystis pneumonia is
not well studied.
Adverse Effects
Primaquine in
recommended doses is generally well tolerated. It infrequently causes nausea,
epigastric pain, abdominal cramps, and headache, and these symptoms are more
common with higher dosages and when the drug is taken on an empty stomach. More
serious but rare adverse effects are leukopenia, agranulocytosis, leukocytosis,
and cardiac arrhythmias. Standard doses of pri-maquine may cause hemolysis or
methemoglobinemia (manifested by cyanosis), especially in persons with G6PD
deficiency or other hereditary metabolic defects.
Contraindications & Cautions
Primaquine should be
avoided in patients with a history of granu-locytopenia or methemoglobinemia,
in those receiving potentially myelosuppressive drugs (eg, quinidine), and in
those with disor-ders that commonly include myelosuppression. It is never given
parenterally because it may induce marked hypotension.
Patients should be
tested for G6PD deficiency before pri-maquine is prescribed. When a patient is
deficient in G6PD, treat-ment strategies may consist of withholding therapy and
treating subsequent relapses, if they occur, with chloroquine; treating
patients with standard dosing, paying close attention to their hema-tologic
status; or treating with weekly primaquine (45 mg base) for 8 weeks.
G6PD-deficient individuals of Mediterranean and Asian ancestry are most likely
to have severe deficiency, whereas those of African ancestry usually have a
milder biochemical defect. This difference can be taken into consideration in
choosing a treatment strategy. In any event, primaquine should be discontinued
if there is evidence of hemolysis or anemia. Primaquine should be avoided in
pregnancy because the fetus is relatively G6PD-deficient and thus at risk of
hemolysis.
Related Topics