Chapter: Basic & Clinical Pharmacology : Antiprotozoal Drugs
Artemisinin & Its Derivatives - Malaria
ARTEMISININ & ITS DERIVATIVES
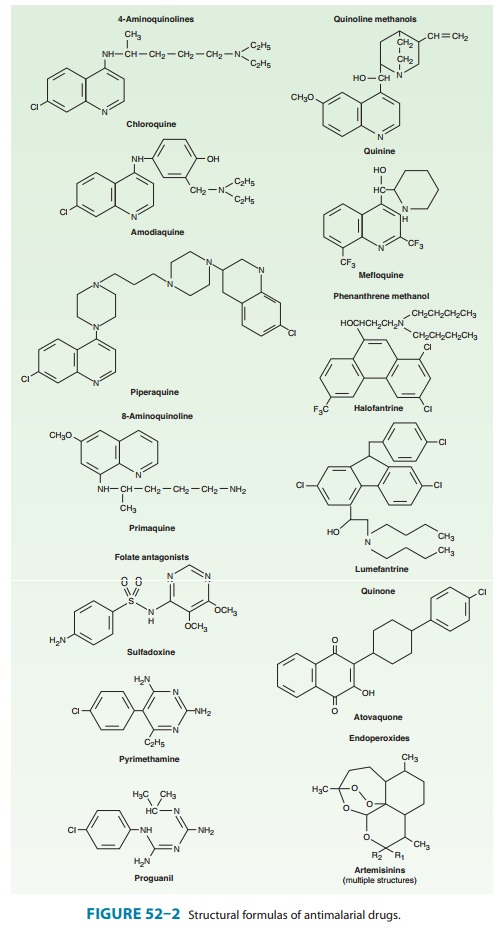
Artemisinin (qinghaosu) is a sesquiterpene lactone
endoperoxide (Figure 52–2), the active component of an herbal medicine that has
been used as an antipyretic in China for over 2000 years. Artemisinin is
insoluble and can only be used orally. Analogs have been synthesized to
increase solubility and improve antimalarial efficacy. The most important of
these analogs are artesunate
(water-soluble; useful for oral, intravenous, intramuscular, and rectal
administration), artemether
(lipid-soluble; useful for oral, intramuscular, and rectal administration), and
dihydroartemisinin (water-soluble; useful
for oral administration).
Chemistry & Pharmacokinetics
Artemisinin and its
analogs are rapidly absorbed, with peak plasma levels occurring in 1–2 hours
and half-lives of 1–3 hours after oral administration. Artemisinin, artesunate,
and artemether are rapidly metabolized to the active metabolite
dihydroartemisi-nin. Drug levels appear to decrease after a number of days of
therapy.
Antimalarial Action & Resistance
The artemisinins are
now widely available around the world. However, artemisinin monotherapy for the
treatment of uncompli-cated malaria is now strongly discouraged. Rather,
co-formulated artemisinin-based combination therapies are recommended to
improve efficacy and prevent the selection of artemisinin-resistant parasites. The
oral combination regimen Coartem (artemether-lumefantrine) was approved by the
Food and Drug Administration (FDA) in 2009, and may be considered the new
first-line therapy in the USA for uncomplicated falciparum malaria, although
the drug may not be widely available. Intravenous artesunate was made available
by the CDC in 2007; use of the drug can be initi-ated by contact with the CDC,
which will release the drug for appropriate indications (falciparum malaria
with signs of severe disease or inability to take oral medications) from stocks
stored around the USA.
Artemisinin and its
analogs are very rapidly acting blood sch-izonticides against all human malaria
parasites. Artemisinins have no effect on hepatic stages. The antimalarial
activity of artemisinins may result from the production of free radicals that
follows the iron-catalyzed cleavage of the artemisinin endoperoxide bridge in
the parasite food vacuole or from inhibition of a parasite calcium ATPase.
Artemisinin resistance is not yet an important problem, but P falciparum isolates with diminished in
vitro susceptibility to arte-mether have recently been described. In addition,
increasing rates of treatment failure and increases in parasite clearance times
after use of artesunate or artesunate-mefloquine in parts of Cambodia may be
early signs of a worrisome decrease in artesunate efficacy.
Clinical Uses
Artemisinin-based
combination therapy is now the standard for treatment of uncomplicated
falciparum malaria in nearly all areas endemic for falciparum malaria. These
regimens were developed because the short plasma half-lives of the artemisinins
led to unac-ceptably high recrudescence rates after short-course therapy, which
were reversed by inclusion of longer-acting drugs. Combination therapy also helps
to protect against the selection of artemisinin resistance. However, with
completion of dosing after 3 days, the artemisinin components are rapidly
eliminated, and so selection of resistance to partner drugs is of concern.
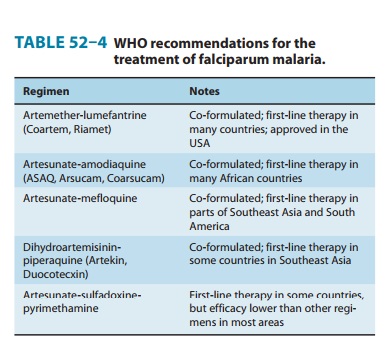
The WHO recommends
five artemisinin-based combinations for the treatment of uncomplicated
falciparum malaria (Table 52–4). One of these,
artesunate-sulfadoxine-pyrimethamine is not rec-ommended in many areas owing to
unacceptable levels of resis-tance to sulfadoxine-pyrimethamine, but it is the
first-line therapy in some countries in Asia, South America, and North Africa.
The other four recommended regimens are now all available as combi-nation
formulations, although manufacturing standards may vary. Artesunate-mefloquine
is highly effective in Southeast Asia, where resistance to many antimalarials
is common; it is the first-line therapy in some countries in Southeast Asia and
South America. This regimen is less practical for other areas, particularly
Africa, because of its relatively high cost and poor tolerability. Either
artesunate-amodiaquine or artemether-lumefantrine is now thestandard
treatment for uncomplicated falciparum malaria in most countries in Africa and
some additional endemic countries on other continents. Dihydroartemisinin-piperaquine
is a newer regi-men that has shown excellent efficacy; it is the first-line
therapy for falciparum malaria in Vietnam.
The relative efficacy
and safety of artemisinin-based combina-tion therapies are now under active
investigation. In general, the leading regimens are highly efficacious, safe,
and well tolerated, and they are the new standard of care for the treatment of
uncom-plicated falciparum malaria.
Artemisinins
are also proving to have outstanding efficacy in the treatment of complicated
falciparum malaria. Large random-ized trials and meta-analyses have shown that
intramuscular artemether has an efficacy equivalent to that of quinine and that
intravenous artesunate is superior to intravenous quinine in terms of parasite
clearance time and—most important—patient sur-vival. Intravenous artesunate
also has a superior side-effect profile compared with that of intravenous
quinine or quinidine. Thus, intravenous artesunate will likely replace quinine
as the standard of care for the treatment of severe falciparum malaria,
although it is not yet widely available in most areas. Artesunate and
arte-mether have also been effective in the treatment of severe malaria when
administered rectally, offering a valuable treatment modality when parenteral
therapy is not available.
Adverse Effects & Cautions
Artemisinins
are generally very well tolerated. The most commonly reported adverse effects
are nausea, vomiting, diarrhea, and dizzi-ness, and these may often be due to
underlying malaria rather than the medications. Rare serious toxicities include
neutropenia, ane-mia, hemolysis, elevated liver enzymes, and allergic
reactions. Irreversible neurotoxicity has been seen in animals, but only after
doses much higher than those used to treat malaria. Artemisinins have been
embryotoxic in animal studies, but rates of congenital abnormalities,
stillbirths, and abortions were not elevated, com-pared with those of controls,
in women who received artemisinins during pregnancy. Based on this information
and the significant risk of malaria during pregnancy, the WHO recommends
artemisinin-based combination therapies for the treatment of uncomplicated
falciparum malaria during the second and third trimesters of preg-nancy,
intravenous artesunate or quinine for the treatment of severe malaria during
the first trimester, and intravenous artesunate for treatment of severe malaria
during the second and third trimesters.
Related Topics